
How do you find the coordination number of sodium chloride (NaCl)?
Answer
524.4k+ views
Hint: The crystal lattice of any molecule is the three – dimensional arrangement of its atoms. In a crystal, the arrangement of atoms is in a way that some atoms surround others. The number of atoms surrounding each atom is a coordination number of that atom. The lattice type of NaCl is Face centered cubic cell.
Complete answer:
The coordination number of any atom is defined as the number of atoms surrounding that atom in its crystal lattice structure. A unit cell is the smallest part of a crystal. A crystal lattice consists of various lattice points, these points are the ions or atoms of the molecule. Unit cells are of primitive (simple) and centered unit types that have FCC, BCC, and ECC.
We have NaCl which is an ionic solid. It is formed in the stoichiometric ratio of 1 : 1 as, 1 sodium atom $N{{a}^{+}}$ is needed for combining by transferring the valence electron to 1 chlorine atom as $C{{l}^{-}}$. This means this cation and anion is situated in the same number and has the same number of ions attached to each of them. As the unit cell of NaCl is FCC, which means that an ion is present at the four corners, and at the 6 faces of the lattice, as the structure of FCC suggests as follows,
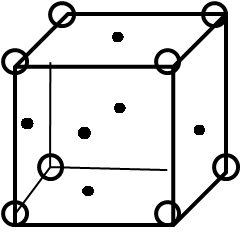
This implies that one $N{{a}^{+}}$ or $C{{l}^{-}}$ ion will be surrounded by ions that are above, below, in the front, at the back, at the right and at the left that makes a total of 6 ions. This means that in NaCl, $N{{a}^{+}}$is surrounded by 6$C{{l}^{-}}$ ions and $C{{l}^{-}}$ is surrounded by 6$N{{a}^{+}}$ ions.
Hence, the coordination number of sodium chloride NaCl is 6.
Note:
The 1 : 1 stoichiometry makes it possess the same coordination number for both atoms. The other unit cells include, BCC that is body centered cell, which has particles at the corners and one at the center. While, ECC called end centered cell has particles on corners and at the two opposite faces.
Complete answer:
The coordination number of any atom is defined as the number of atoms surrounding that atom in its crystal lattice structure. A unit cell is the smallest part of a crystal. A crystal lattice consists of various lattice points, these points are the ions or atoms of the molecule. Unit cells are of primitive (simple) and centered unit types that have FCC, BCC, and ECC.
We have NaCl which is an ionic solid. It is formed in the stoichiometric ratio of 1 : 1 as, 1 sodium atom $N{{a}^{+}}$ is needed for combining by transferring the valence electron to 1 chlorine atom as $C{{l}^{-}}$. This means this cation and anion is situated in the same number and has the same number of ions attached to each of them. As the unit cell of NaCl is FCC, which means that an ion is present at the four corners, and at the 6 faces of the lattice, as the structure of FCC suggests as follows,

This implies that one $N{{a}^{+}}$ or $C{{l}^{-}}$ ion will be surrounded by ions that are above, below, in the front, at the back, at the right and at the left that makes a total of 6 ions. This means that in NaCl, $N{{a}^{+}}$is surrounded by 6$C{{l}^{-}}$ ions and $C{{l}^{-}}$ is surrounded by 6$N{{a}^{+}}$ ions.
Hence, the coordination number of sodium chloride NaCl is 6.
Note:
The 1 : 1 stoichiometry makes it possess the same coordination number for both atoms. The other unit cells include, BCC that is body centered cell, which has particles at the corners and one at the center. While, ECC called end centered cell has particles on corners and at the two opposite faces.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




