
How do you find the geometry of $TeC{{l}_{4}}$ using the VSEPR method?
Answer
560.4k+ views
Hint: There are 2 valence electrons in Te and 7 valence electrons in Cl.
The Te is the central metal atom in the compound
Complete step by step answer:
- So in the question, it is asked how will we find the geometry of $TeC{{l}_{4}}$ using the VSEPR method. So first we have to know what the VSEPR method means and then let’s discuss the steps by which we attain the geometry of our molecule.
-VSEPR theory means Valence shell Electron Pair Repulsion theory, as the abbreviation states there will be repulsion between the electrons present in the atoms, hence the atom will tend to move apart and tend to attain a geometry with minimum electronic repulsion.
- We use VSEPR theory for finding the geometry and the shape of the compounds given.
So let’s discuss the step by step in which we get the final geometry and shape of the compounds by solving the problem given in the question.
- Here we are discussing $TeC{{l}_{4}}$ and we have to find the geometry and the shape of the molecule.
- First we should calculate the total valence electrons present in the compound .For that we should know the valence electrons of different atoms in the compound.
- The element Te belongs to the oxygen family and hence it has a valence electrons of 6 and the Cl belongs to the group-17 which has 7 valence electrons in their valence shell.
- Now let’s calculate the number of valence electrons in the compound.
$\text{Valence}\,\text{electrons}\,\text{in}\,\text{TeC}{{\text{l}}_{\text{4}}}\text{=6+4 }\!\!\times\!\!\text{ }\left( \text{7} \right)=34{{e}^{-}}s$
- So there are 34 valence electrons in the given molecule and let's draw the Lewis structure for the molecule so that we will get an idea of the geometry and the number of bond pairs and lone pairs in the central atom.
- Here the least electronegative atom is Te and it is taken as the central metal atom and the four chlorine atoms are placed surrounding the Te atom.
And then distribute the electrons in such a fashion that all the atoms will have octet configuration.
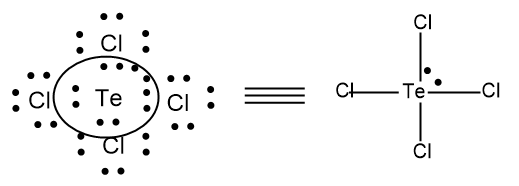
- Here we can see a lone pair on the central atom, since there are 34 valence electrons present and to complete the octet configuration in $TeC{{l}_{4}}$, we only need 32 electrons and hence there is extra 2 electrons present and it is arranged along with the central atom and the central atom has five pairs of electrons in which four pairs are used to bond with 4 Cl atoms.
- So there is 4 bond pairs and 1 lone pair of electrons and the molecule $TeC{{l}_{4}}$ belongs molecules having the formulae $\text{A}{{\text{B}}_{\text{4}}}\text{E}$, in which A denotes the central atom B denotes the number of bond pairs and E denotes the number of bond pairs.
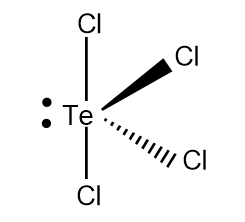
- In VSEPR theory a molecule which belongs to formula group of $\text{A}{{\text{B}}_{\text{4}}}\text{E}$, will have the electron pair geometry of trigonal bipyramidal and since they have one lone pair in them the trigonal bipyramidal geometry of the molecule is distorted to see-saw geometry, with bond angles ${{90}^{\circ }},{{120}^{\circ }}$ and ${{180}^{\circ }}$. But since the Te possesses a lone pair, there will be a repulsion between the bond pair and the lone pair which leads to the slight variation in the bond angle of the molecule .
The final see-saw structure of $TeC{{l}_{4}}$ can be drawn as,
Note: We can find the number of electron pairs around the central atom of a molecule if we know the number of valence electrons in the central atom and the number of atoms attached to the central atom through single bonds.
$\text{No}\text{.}\,\text{of}\,{{\text{e}}^{\text{-}}}\text{pairs}\,\text{around}\,\text{central}\,\text{atom}$
$=\dfrac{1}{2}$$\left( \text{No}\text{. of valence electrons in central atom + No}\text{.}\,\text{of}\,\text{sigma bonds in the molecule} \right)$
The shape of the molecule depends on the number of lone pairs and bond pairs in the molecule, since the atoms will arrange in such a way to obtain the maximum stability and minimize the repulsion between the lone pairs and bonds pairs depending on their number.
Example: The molecule having geometry $A{{B}_{2}}{{E}_{2}}$, here E represents the number of lone pair, B represents bond pairs and A is the central atom will have bent shape, whereas the molecule type $A{{B}_{2}}$ (only bond pairs) will be linear in shape.
The Te is the central metal atom in the compound
Complete step by step answer:
- So in the question, it is asked how will we find the geometry of $TeC{{l}_{4}}$ using the VSEPR method. So first we have to know what the VSEPR method means and then let’s discuss the steps by which we attain the geometry of our molecule.
-VSEPR theory means Valence shell Electron Pair Repulsion theory, as the abbreviation states there will be repulsion between the electrons present in the atoms, hence the atom will tend to move apart and tend to attain a geometry with minimum electronic repulsion.
- We use VSEPR theory for finding the geometry and the shape of the compounds given.
So let’s discuss the step by step in which we get the final geometry and shape of the compounds by solving the problem given in the question.
- Here we are discussing $TeC{{l}_{4}}$ and we have to find the geometry and the shape of the molecule.
- First we should calculate the total valence electrons present in the compound .For that we should know the valence electrons of different atoms in the compound.
- The element Te belongs to the oxygen family and hence it has a valence electrons of 6 and the Cl belongs to the group-17 which has 7 valence electrons in their valence shell.
- Now let’s calculate the number of valence electrons in the compound.
$\text{Valence}\,\text{electrons}\,\text{in}\,\text{TeC}{{\text{l}}_{\text{4}}}\text{=6+4 }\!\!\times\!\!\text{ }\left( \text{7} \right)=34{{e}^{-}}s$
- So there are 34 valence electrons in the given molecule and let's draw the Lewis structure for the molecule so that we will get an idea of the geometry and the number of bond pairs and lone pairs in the central atom.
- Here the least electronegative atom is Te and it is taken as the central metal atom and the four chlorine atoms are placed surrounding the Te atom.
And then distribute the electrons in such a fashion that all the atoms will have octet configuration.

- Here we can see a lone pair on the central atom, since there are 34 valence electrons present and to complete the octet configuration in $TeC{{l}_{4}}$, we only need 32 electrons and hence there is extra 2 electrons present and it is arranged along with the central atom and the central atom has five pairs of electrons in which four pairs are used to bond with 4 Cl atoms.
- So there is 4 bond pairs and 1 lone pair of electrons and the molecule $TeC{{l}_{4}}$ belongs molecules having the formulae $\text{A}{{\text{B}}_{\text{4}}}\text{E}$, in which A denotes the central atom B denotes the number of bond pairs and E denotes the number of bond pairs.
- In VSEPR theory a molecule which belongs to formula group of $\text{A}{{\text{B}}_{\text{4}}}\text{E}$, will have the electron pair geometry of trigonal bipyramidal and since they have one lone pair in them the trigonal bipyramidal geometry of the molecule is distorted to see-saw geometry, with bond angles ${{90}^{\circ }},{{120}^{\circ }}$ and ${{180}^{\circ }}$. But since the Te possesses a lone pair, there will be a repulsion between the bond pair and the lone pair which leads to the slight variation in the bond angle of the molecule .
The final see-saw structure of $TeC{{l}_{4}}$ can be drawn as,

Note: We can find the number of electron pairs around the central atom of a molecule if we know the number of valence electrons in the central atom and the number of atoms attached to the central atom through single bonds.
$\text{No}\text{.}\,\text{of}\,{{\text{e}}^{\text{-}}}\text{pairs}\,\text{around}\,\text{central}\,\text{atom}$
$=\dfrac{1}{2}$$\left( \text{No}\text{. of valence electrons in central atom + No}\text{.}\,\text{of}\,\text{sigma bonds in the molecule} \right)$
The shape of the molecule depends on the number of lone pairs and bond pairs in the molecule, since the atoms will arrange in such a way to obtain the maximum stability and minimize the repulsion between the lone pairs and bonds pairs depending on their number.
Example: The molecule having geometry $A{{B}_{2}}{{E}_{2}}$, here E represents the number of lone pair, B represents bond pairs and A is the central atom will have bent shape, whereas the molecule type $A{{B}_{2}}$ (only bond pairs) will be linear in shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




