
How do you find the molar mass of argon?
Answer
565.2k+ views
Hint Molar mass is roughly equal to the weight of the nucleus.
The molar mass of Ar is not a whole number and the atomic number of Ar is 18.
Complete answer:
So in the question it is asked how we will determine or calculate the molar mass of Argon.
From the time we are learning the periodic table we are very much familiar with the two terms about an element, the atomic number and the molar mass of the element.
Atomic number is the unique number for an element which is the identity for an element. No two elements will possess the same atomic number. The atomic number gives the number of protons and the number of electrons present in the elements, since the atomic number will be equal to the number of protons and the number of electrons in the element.
Here we are concerned about the Ar element and it has an atomic number of 18.So the number of protons and electrons present are also 18.
Now we will discuss the molar mass or atomic weight of an element. The atomic weight is equal to the weight of the nucleus of the atom. Atomic mass is equal to the sum of protons and neutrons present in the nucleus.
We can easily find the molar mass with the aid of a periodic table, the atomic mass will be given in the left bottom of the chemical symbol of the element in the periodic table.
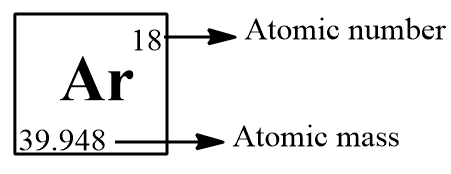
Here the atomic mass number is not a whole number. For many elements the atomic mass is not having a whole number since these elements have isotopes and the elements exist as the combination of these isotopes and we take the sum of the average mass of the isotopes as the molar mass of the element. Ar possess 26 isotopes but the most stable three isotopes present in Earth are: $A{{r}^{36}},A{{r}^{38}}$and $A{{r}^{40}}$ and we take the average mass of these isotopes to get the molar mass of Ar and we will get a value of 39.948.
Note: We can also find the value of atomic mass if the number of neutrons and protons are present in an atom it will be roughly equal to the atomic mass since many atoms exist as the combination of their isotopes in the real world.
If the number of moles of Ar in the taken sample and the weight of the sample is given then we could find the molar mass of Ar by altering the equation,
$\text{No}\text{.}\,\text{of}\,\text{moles}\,\text{=}\dfrac{\text{Given}\,\text{mass}}{\text{Molar}\,\text{mass}}$
$\text{Molar}\,\text{mass=}\dfrac{\text{Given}\,\text{mass}}{\text{No}\text{.}\,\text{of}\,\text{moles}}$
Molar mass is also called molecular mass, molecular weight gram formula mass etc.
The unit of molecular mass is $\text{g/mol}$
The molar mass of Ar is not a whole number and the atomic number of Ar is 18.
Complete answer:
So in the question it is asked how we will determine or calculate the molar mass of Argon.
From the time we are learning the periodic table we are very much familiar with the two terms about an element, the atomic number and the molar mass of the element.
Atomic number is the unique number for an element which is the identity for an element. No two elements will possess the same atomic number. The atomic number gives the number of protons and the number of electrons present in the elements, since the atomic number will be equal to the number of protons and the number of electrons in the element.
Here we are concerned about the Ar element and it has an atomic number of 18.So the number of protons and electrons present are also 18.
Now we will discuss the molar mass or atomic weight of an element. The atomic weight is equal to the weight of the nucleus of the atom. Atomic mass is equal to the sum of protons and neutrons present in the nucleus.
We can easily find the molar mass with the aid of a periodic table, the atomic mass will be given in the left bottom of the chemical symbol of the element in the periodic table.

Here the atomic mass number is not a whole number. For many elements the atomic mass is not having a whole number since these elements have isotopes and the elements exist as the combination of these isotopes and we take the sum of the average mass of the isotopes as the molar mass of the element. Ar possess 26 isotopes but the most stable three isotopes present in Earth are: $A{{r}^{36}},A{{r}^{38}}$and $A{{r}^{40}}$ and we take the average mass of these isotopes to get the molar mass of Ar and we will get a value of 39.948.
Note: We can also find the value of atomic mass if the number of neutrons and protons are present in an atom it will be roughly equal to the atomic mass since many atoms exist as the combination of their isotopes in the real world.
If the number of moles of Ar in the taken sample and the weight of the sample is given then we could find the molar mass of Ar by altering the equation,
$\text{No}\text{.}\,\text{of}\,\text{moles}\,\text{=}\dfrac{\text{Given}\,\text{mass}}{\text{Molar}\,\text{mass}}$
$\text{Molar}\,\text{mass=}\dfrac{\text{Given}\,\text{mass}}{\text{No}\text{.}\,\text{of}\,\text{moles}}$
Molar mass is also called molecular mass, molecular weight gram formula mass etc.
The unit of molecular mass is $\text{g/mol}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




