
Find the number of lone pairs and bond pairs in$S{{F}_{6}}$.
Answer
585.6k+ views
Hint: The number of lone pair and bond pair can be calculated by identifying the Lewis dot structure of the given molecule. The number of lone pairs added with the number of bond pairs is equivalent to the valence electron around the atom.
Complete step by step solution:
Before calculating the number of lone pair and bond pair let’s understand what lone pair and bond pair means. A lone pair means a pair of valence electrons which is not shared with another atom. It is also known as an unshared pair or nonbonding pair of electrons.
When two atoms combine to form a covalent bond electrons are shared between both the atoms, these electrons which are present in the covalent bond are known as bond pairs.
Let’s start calculating the number of lone pair and bond pair by drawing the Lewis dot structure of $S{{F}_{6}}$.
First, we need to count the valence electrons, we can calculate it using the periodic table. Sulfur has 6 electrons in the valence shell and fluorine has 7 electrons in the valence shell.
Total valence electrons in $S{{F}_{6}}$= $6+6\left( 7 \right)=48$ valence electrons.
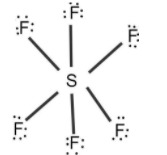
From the Lewis dot structure, we can conclude that there is 18 lone pair (3 on each fluorine and 0 on sulfur) and 6 bond pairs in the $S{{F}_{6}}$molecule.
Note: Note that sulfur can have expanded octet which means that it can have more than 8 valence electrons in the outermost shell. So for the structure of $S{{F}_{6}}$ there are 12 valence electrons on sulfur and 8 valence electrons on fluorine.
Complete step by step solution:
Before calculating the number of lone pair and bond pair let’s understand what lone pair and bond pair means. A lone pair means a pair of valence electrons which is not shared with another atom. It is also known as an unshared pair or nonbonding pair of electrons.
When two atoms combine to form a covalent bond electrons are shared between both the atoms, these electrons which are present in the covalent bond are known as bond pairs.
Let’s start calculating the number of lone pair and bond pair by drawing the Lewis dot structure of $S{{F}_{6}}$.
First, we need to count the valence electrons, we can calculate it using the periodic table. Sulfur has 6 electrons in the valence shell and fluorine has 7 electrons in the valence shell.
Total valence electrons in $S{{F}_{6}}$= $6+6\left( 7 \right)=48$ valence electrons.

From the Lewis dot structure, we can conclude that there is 18 lone pair (3 on each fluorine and 0 on sulfur) and 6 bond pairs in the $S{{F}_{6}}$molecule.
Note: Note that sulfur can have expanded octet which means that it can have more than 8 valence electrons in the outermost shell. So for the structure of $S{{F}_{6}}$ there are 12 valence electrons on sulfur and 8 valence electrons on fluorine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




