
Find the number of unpaired electrons in (a) P, (b) Si, (c) Cr, (d) Fe and (e) Kr.
Answer
522.8k+ views
Hint: We shall write the electronic configurations of each of these atoms
one by one, then we can figure out the number of unpaired electrons by drawing
the box diagram for the outer electronic configuration of each element.
Complete answer:
> Let us begin with phosphorus. Its atomic number is 15. So its electronic configuration
will be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$ . The p-subshell has 3 orbitals and the electrons fill in accordance with Hund’s rule of maximum multiplicity, that is, each degenerate orbital (meaning in cases of orbitals having same energy) is singly occupied by an electron and only after all the orbitals are singly occupied, electron pairing occurs. When we look at the outer electronic configuration of phosphorus, it is $3{s^2}3{p^3}$ so if we represent it using box figures, it would appear as shown below:
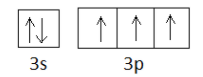
As you can see, the number of unpaired electrons present is 3.
> Moving on to silicon, again, follow the same steps. Its atomic number is 14, so let us write its electronic configuration. It will be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^2}$ . So in this case also, the configuration is ending at 3p. We already know that the p subshell contains 3 orbitals. So let us take a look at the box figure of the outer electronic configuration of silicon.
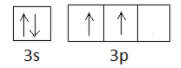
So the number of unpaired electrons in silicon is 2.
> Similarly, let us determine the number of unpaired electrons in Cr. Chromium has atomic number 24 and its electronic configuration is quite unique. We expect its configuration to be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^4}$ but actually, its electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}3{d^5}$ . This happens because half-filled orbitals account for stability. The maximum capacity of s-subshell which contains only one orbital is 2 electrons and the maximum capacity of d-subshell that contains five orbitals is 10 electrons. In the configuration of Cr, you can see that both 4s and 3d are half-filled, which leads to stability. So the outer electronic configuration is $4{s^1}3{d^5}$ which can be represented using box figures as
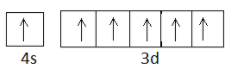
So the total number of unpaired electrons in Cr is 6.
By now, you must have got a hang of how to go about. Let us determine the number of unpaired electrons in Fe now. Iron with atomic number 26 has the electronic configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^6}$ . The box figure of the outer electronic configuration would appear as:
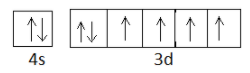
So the number of unpaired electrons in Fe is 4.
> Unlike what we have done so far, we need not write the electronic configuration of Kr to figure out the number of unpaired electrons that it contains. We know that krypton is a noble gas. In all noble gases, the number of unpaired electrons will be zero.
Note: Just like Cr, the electronic configuration of Cu is also different from what it is expected to be. The correct electronic configuration of Cu with atomic number 29 is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}3{d^{10}}$ . So here, as you can see, 4s is half filled and 3d is fully filled and this leads to stability. Keep in mind that half-filled orbitals and fully filled orbitals are stable.
one by one, then we can figure out the number of unpaired electrons by drawing
the box diagram for the outer electronic configuration of each element.
Complete answer:
> Let us begin with phosphorus. Its atomic number is 15. So its electronic configuration
will be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$ . The p-subshell has 3 orbitals and the electrons fill in accordance with Hund’s rule of maximum multiplicity, that is, each degenerate orbital (meaning in cases of orbitals having same energy) is singly occupied by an electron and only after all the orbitals are singly occupied, electron pairing occurs. When we look at the outer electronic configuration of phosphorus, it is $3{s^2}3{p^3}$ so if we represent it using box figures, it would appear as shown below:

As you can see, the number of unpaired electrons present is 3.
> Moving on to silicon, again, follow the same steps. Its atomic number is 14, so let us write its electronic configuration. It will be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^2}$ . So in this case also, the configuration is ending at 3p. We already know that the p subshell contains 3 orbitals. So let us take a look at the box figure of the outer electronic configuration of silicon.

So the number of unpaired electrons in silicon is 2.
> Similarly, let us determine the number of unpaired electrons in Cr. Chromium has atomic number 24 and its electronic configuration is quite unique. We expect its configuration to be $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^4}$ but actually, its electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}3{d^5}$ . This happens because half-filled orbitals account for stability. The maximum capacity of s-subshell which contains only one orbital is 2 electrons and the maximum capacity of d-subshell that contains five orbitals is 10 electrons. In the configuration of Cr, you can see that both 4s and 3d are half-filled, which leads to stability. So the outer electronic configuration is $4{s^1}3{d^5}$ which can be represented using box figures as

So the total number of unpaired electrons in Cr is 6.
By now, you must have got a hang of how to go about. Let us determine the number of unpaired electrons in Fe now. Iron with atomic number 26 has the electronic configuration $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^6}$ . The box figure of the outer electronic configuration would appear as:

So the number of unpaired electrons in Fe is 4.
> Unlike what we have done so far, we need not write the electronic configuration of Kr to figure out the number of unpaired electrons that it contains. We know that krypton is a noble gas. In all noble gases, the number of unpaired electrons will be zero.
Note: Just like Cr, the electronic configuration of Cu is also different from what it is expected to be. The correct electronic configuration of Cu with atomic number 29 is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}3{d^{10}}$ . So here, as you can see, 4s is half filled and 3d is fully filled and this leads to stability. Keep in mind that half-filled orbitals and fully filled orbitals are stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




