
Find the oxidation number of carbon in HCHO.
Answer
598.8k+ views
Hint: Draw the structure of the given hydrocarbon. Calculate the oxidation number by summing the oxidation number of each element to 0 since this element has a net charge equal to zero. Assign a random variable ‘x’ for calculating oxidation number of C.
Complete step by step answer:
Let us begin by drawing the structure of HCHO.
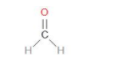
The compound HCHO is called methanol. This is neutral and therefore we can say it has a net charge = 0.
Hydrogen has an oxidation state of +1.
Oxygen has an oxidation state of -2, since it has 6 electrons in its valence shell.
Let the oxidation number of Carbon be ‘x’.
Equating oxidation state of each element with the oxidation state of the compound,
2(+1) + x + (-2) = 0
x = 0.
Therefore, the oxidation number of carbon in HCHO is 0.
Additional Information: The compound methanol is also known as Formaldehyde. It is the simplest aldehyde.
Note: The oxidation number of an atom or element is a number that indicates the total number of electrons lost or gained by it. There are certain rules for calculating oxidation number –
-Oxidation number of any free element is zero.
-Oxidation number for a monatomic ion is equal to the net charge on it.
-Hydrogen, in general, has an oxidation state equal to +1. However, it is -1 in forms of a compound with an element with lesser electronegativity.
-Alkali metals have oxidation number = +1
-Alkali earth metals have oxidation number = +2
-Halogens have oxidation number = -1
-Oxygen has an oxidation number = -2, but in case of peroxides, it is -1.
Complete step by step answer:
Let us begin by drawing the structure of HCHO.

The compound HCHO is called methanol. This is neutral and therefore we can say it has a net charge = 0.
Hydrogen has an oxidation state of +1.
Oxygen has an oxidation state of -2, since it has 6 electrons in its valence shell.
Let the oxidation number of Carbon be ‘x’.
Equating oxidation state of each element with the oxidation state of the compound,
2(+1) + x + (-2) = 0
x = 0.
Therefore, the oxidation number of carbon in HCHO is 0.
Additional Information: The compound methanol is also known as Formaldehyde. It is the simplest aldehyde.
Note: The oxidation number of an atom or element is a number that indicates the total number of electrons lost or gained by it. There are certain rules for calculating oxidation number –
-Oxidation number of any free element is zero.
-Oxidation number for a monatomic ion is equal to the net charge on it.
-Hydrogen, in general, has an oxidation state equal to +1. However, it is -1 in forms of a compound with an element with lesser electronegativity.
-Alkali metals have oxidation number = +1
-Alkali earth metals have oxidation number = +2
-Halogens have oxidation number = -1
-Oxygen has an oxidation number = -2, but in case of peroxides, it is -1.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




