
Find the plane of symmetry, center of symmetry and axis of symmetry in the following molecules
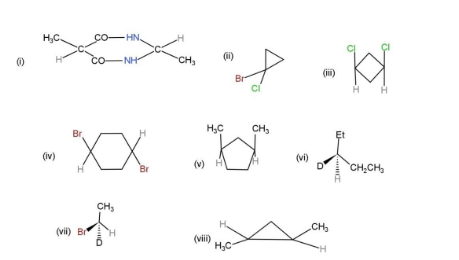

Answer
534.6k+ views
Hint: Symmetry of a molecule is the orientation of the substituted, or the alkyl groups, on that molecule, such that it seems to be a mirror image when the molecule is cut into half.
Complete step by step solution: Plane of symmetry for a molecule is the imaginary division, through which the molecule is bilaterally divided into two equal halves. These halves are mirror images of one another.
Center of symmetry of a molecule is the center point from which the identical atoms exist on the opposite side from this center at equal distance.
An axis of symmetry is the line along which a molecule is considered to be half, and when rotated from this axis, it is the same molecule from both the sides.
From the given molecules, only 3 molecules consist of a plane of symmetry, center, and axis of symmetry. Other molecules when cut into two halves, are not the mirror images of each other, so, they cannot be considered as symmetrical. The molecules with plane, center and axis of symmetry are shown as,
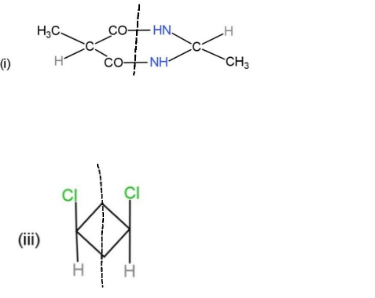
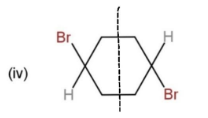
These three molecules contain symmetry and the planes and center are shown through a line dividing them.
Hence, molecules, (i), (iii), and (iv), show symmetry.
Note: Other compounds have no plane of symmetry, as they cannot be divided into mirror images with equal alkyl groups, carbon atoms, or any other substituted group. Although some of them have a chiral center, they cannot be divided into equal halves.
Complete step by step solution: Plane of symmetry for a molecule is the imaginary division, through which the molecule is bilaterally divided into two equal halves. These halves are mirror images of one another.
Center of symmetry of a molecule is the center point from which the identical atoms exist on the opposite side from this center at equal distance.
An axis of symmetry is the line along which a molecule is considered to be half, and when rotated from this axis, it is the same molecule from both the sides.
From the given molecules, only 3 molecules consist of a plane of symmetry, center, and axis of symmetry. Other molecules when cut into two halves, are not the mirror images of each other, so, they cannot be considered as symmetrical. The molecules with plane, center and axis of symmetry are shown as,


These three molecules contain symmetry and the planes and center are shown through a line dividing them.
Hence, molecules, (i), (iii), and (iv), show symmetry.
Note: Other compounds have no plane of symmetry, as they cannot be divided into mirror images with equal alkyl groups, carbon atoms, or any other substituted group. Although some of them have a chiral center, they cannot be divided into equal halves.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




