
Who was the first scientist to propose a model for the structure of an atom \[?\]
(A) J.J. Thomson
(B) Dalton
(C) Ernest Rutherford
(D) E. Goldstein
Answer
513.9k+ views
Hint :Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding. Although Thomson’s model explained that atoms are electrically neutral, the results of experiments carried out by other scientists could not be explained by this model.
Complete Step By Step Answer:
Thomson model of atom can also be thought of as a watermelon, the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon (figure).
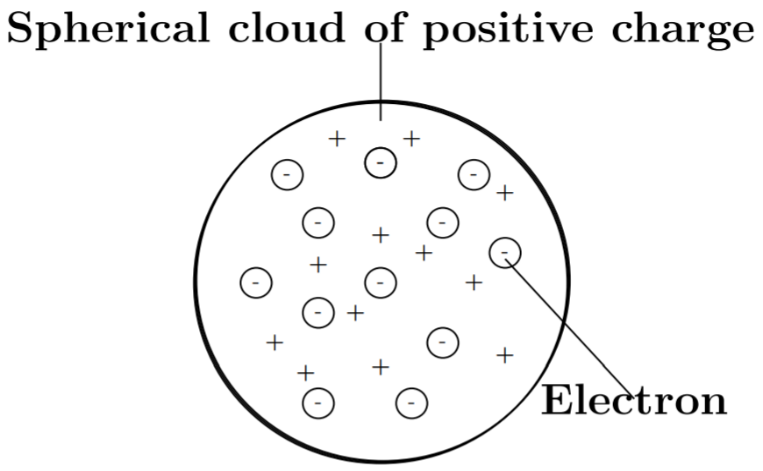
Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. Many scientists proposed various atomic models for explaining this.
J.J. Thomson was the first scientist to propose a model for the structure of an atom.
Hence, the Option (A) is correct.
Note :
Thomson proposed that:
(i) An atom consists of a positively charged sphere and the electrons are embedded in it.
(ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Complete Step By Step Answer:
Thomson model of atom can also be thought of as a watermelon, the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon (figure).

Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. Many scientists proposed various atomic models for explaining this.
J.J. Thomson was the first scientist to propose a model for the structure of an atom.
Hence, the Option (A) is correct.
Note :
Thomson proposed that:
(i) An atom consists of a positively charged sphere and the electrons are embedded in it.
(ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




