
Fluorine molecules are formed by
A) The axial p-p orbital overlap
B) The sideways p-p orbital overlap
C) The s-s orbital overlap
D) The s-p orbital overlap
Answer
501.3k+ views
Hint: Fluorine molecule is bonded by strong sigma bond. This type of covalent bond is formed by the head-to-head overlap of bonding orbitals along the internuclear axis. This is known as axial overlap or head-on overlap. p-p overlapping takes place when two half-filled p-orbitals of the two atoms approach each other.
Complete answer:
Before going to explain this solution. We will go with each option.
(a) The axial p-p orbital overlap:
In fluorine $ 2{p_z} $ orbitals are partially filled they will overlap in head to head fashion. Hence strong sigma bonds will be formed. Fluorine electronic configuration is $ 1{s^2}2{s^2}2{p^5} $ . So the last electron will go in $ {p_z} $ orbital.
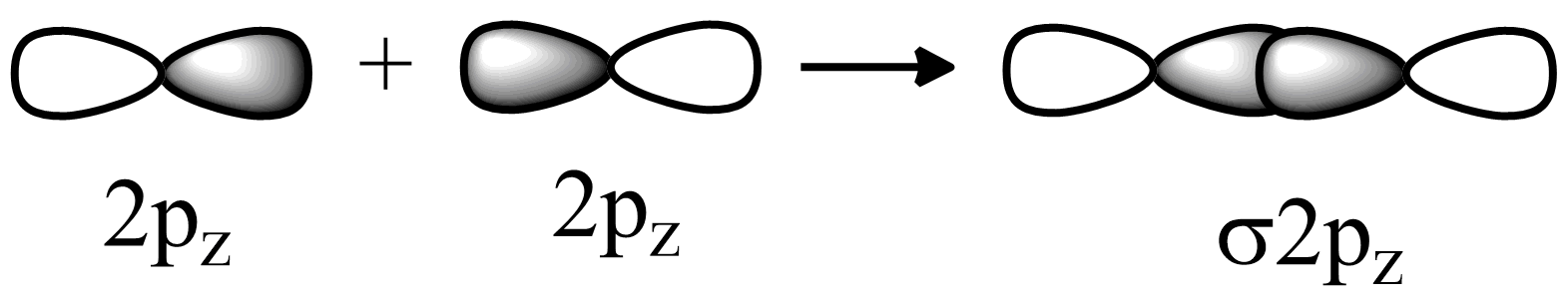
(b) The sideways p-p orbital overlap-
This type of overlapping is possible in $ {p_x} $ and $ {p_y} $ orbital. These orbits undergo sideways overlapping and the $ {p_z} $ orbital will undergo head to head overlapping. Fluorine molecules will not undergo sideways p-p orbital overlap.
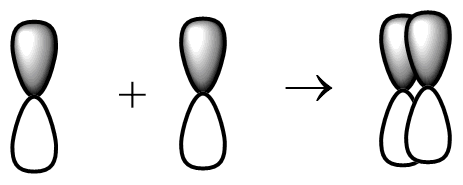
The rest of the overlapping is not possible in fluorine molecule as we can see that fluorine molecule as $ 9 $ atomic number so its electronic configuration will be like $ 1{s^2}2{s^2}2{p^5} $ . In fluorine, there is $ 2p $ partially filled orbital, so fluorine molecules come to each other to overlap and this overlapping of molecules will result in a covalent bond.
Hence, the correct option is (A).
Note:
The strength of a bond depends upon the extent of overlapping. As we can see that fluorine molecule has $ 2{p^5} $ partially-filled orbital, if we will consider this $ p $ orbital as normal $ 2p $ orbital instead of $ 2{p_z} $ orbital then we will make mistake and will choose the wrong option.
Complete answer:
Before going to explain this solution. We will go with each option.
(a) The axial p-p orbital overlap:
In fluorine $ 2{p_z} $ orbitals are partially filled they will overlap in head to head fashion. Hence strong sigma bonds will be formed. Fluorine electronic configuration is $ 1{s^2}2{s^2}2{p^5} $ . So the last electron will go in $ {p_z} $ orbital.
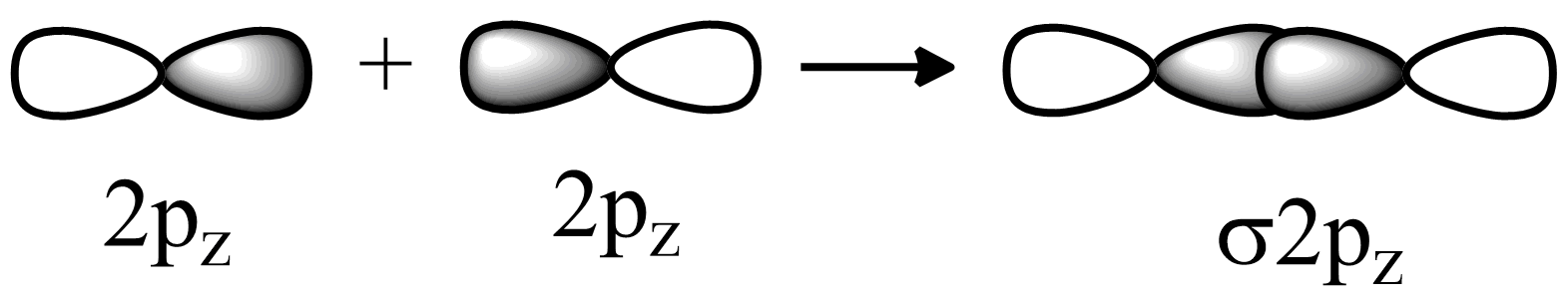
(b) The sideways p-p orbital overlap-
This type of overlapping is possible in $ {p_x} $ and $ {p_y} $ orbital. These orbits undergo sideways overlapping and the $ {p_z} $ orbital will undergo head to head overlapping. Fluorine molecules will not undergo sideways p-p orbital overlap.
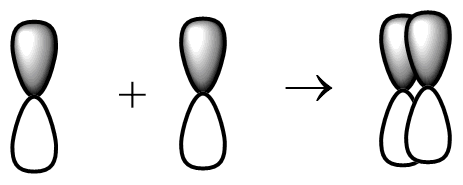
The rest of the overlapping is not possible in fluorine molecule as we can see that fluorine molecule as $ 9 $ atomic number so its electronic configuration will be like $ 1{s^2}2{s^2}2{p^5} $ . In fluorine, there is $ 2p $ partially filled orbital, so fluorine molecules come to each other to overlap and this overlapping of molecules will result in a covalent bond.
Hence, the correct option is (A).
Note:
The strength of a bond depends upon the extent of overlapping. As we can see that fluorine molecule has $ 2{p^5} $ partially-filled orbital, if we will consider this $ p $ orbital as normal $ 2p $ orbital instead of $ 2{p_z} $ orbital then we will make mistake and will choose the wrong option.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




