
For a second order, the graph plotted between ${{[A]}^{-1}}$and time ‘t’ is shown here. $\theta ={{\tan }^{-1}}(0.5)$ and OP = $2Lmo{{l}^{-1}}$. Thus, rate of the reaction at start is:
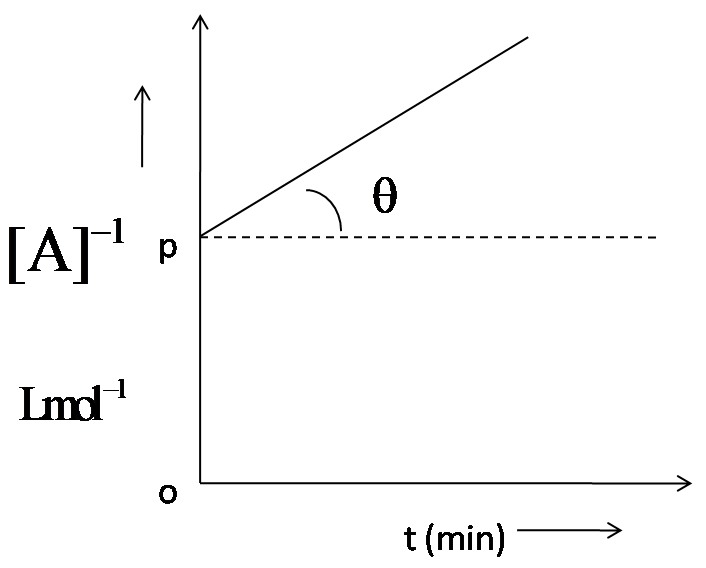
A.$125\,Lmo{{l}^{-1}}{{\min }^{-1}}$
B.$0.124\,Lmo{{l}^{-1}}{{\min }^{-1}}$
C.$1.25\,mol\,Lmo{{l}^{-1}}{{\min }^{-1}}$
D.$0.5\,mol\,Lmo{{l}^{-1}}{{\min }^{-1}}$
Answer
531k+ views
Hint: The reaction in which the concentration of the species that are reacting is raised to the power of two is the second order reaction. Rate constant of any reaction is the relationship between concentration of the reactants and the rate. Rate constant of the second order reaction has unit $mo{{l}^{-1}}L{{\sec }^{-1}}$.
Complete answer:
We have been given a graph plotted between $\dfrac{1}{[A]}$ and time ‘t’. It is a second order reaction. We have to determine the rate of the reaction at the start.
So, For this second order reaction (involving one reactant A), the equation will be $2A\to products$. Applying the law of mass action, the rate of this reaction will be, $\dfrac{dx}{dt}\propto {{[A]}^{2}}$
So, changing the proportionality with rate constant k, we have,
$\dfrac{dx}{dt}=k{{[A]}^{2}}$, this equation can be rewritten as,
$\dfrac{dx}{{{[A]}^{2}}}=kdt$
Integrating the above equation we have,
$\int{\dfrac{dx}{{{[A]}^{2}}}=\int{kdt}}$ this will be,
$\dfrac{{{[A]}^{-1}}}{-1}\times (-1)=kt+I$
$\dfrac{1}{{{[A]}^{-1}}}=kt+I$ , where $I$ is the constant of integration. In the beginning, time, t = 0, and the reactant will be A only, as it has not been reacted.
This equation resembles the equation of a line, slope- intercept form, which is y = mx + b, where m is the slope, x is the x coordinate, y is the y coordinate, and b is the y intercept.
The second order reaction has led to $\dfrac{1}{{{[A]}^{-1}}}=kt+I$, from which it is clear that the slope of the graph gives us rate constant, while y coordinate gives $\dfrac{1}{[A]}$and x coordinate time, ‘t’. so, the slope of the straight line which is $\tan \theta $ will give us the rate. As given, $\theta ={{\tan }^{-1}}=0.5$, this will be the rate of the reaction.
Hence, the rate of the reaction at the start is $0.5\,mol\,Lmo{{l}^{-1}}{{\min }^{-1}}$, so option D is correct.
Note:
The derivation explained is the integrated form of rate equation, rate equation states that rate is directly proportional to the concentration of reactants. Through the integration of rate equation for second order reaction, we can obtain the slope- intercept form equation, which can tell us the value of rate constant, which is the slope of the graph between concentration and time.
Complete answer:
We have been given a graph plotted between $\dfrac{1}{[A]}$ and time ‘t’. It is a second order reaction. We have to determine the rate of the reaction at the start.
So, For this second order reaction (involving one reactant A), the equation will be $2A\to products$. Applying the law of mass action, the rate of this reaction will be, $\dfrac{dx}{dt}\propto {{[A]}^{2}}$
So, changing the proportionality with rate constant k, we have,
$\dfrac{dx}{dt}=k{{[A]}^{2}}$, this equation can be rewritten as,
$\dfrac{dx}{{{[A]}^{2}}}=kdt$
Integrating the above equation we have,
$\int{\dfrac{dx}{{{[A]}^{2}}}=\int{kdt}}$ this will be,
$\dfrac{{{[A]}^{-1}}}{-1}\times (-1)=kt+I$
$\dfrac{1}{{{[A]}^{-1}}}=kt+I$ , where $I$ is the constant of integration. In the beginning, time, t = 0, and the reactant will be A only, as it has not been reacted.
This equation resembles the equation of a line, slope- intercept form, which is y = mx + b, where m is the slope, x is the x coordinate, y is the y coordinate, and b is the y intercept.
The second order reaction has led to $\dfrac{1}{{{[A]}^{-1}}}=kt+I$, from which it is clear that the slope of the graph gives us rate constant, while y coordinate gives $\dfrac{1}{[A]}$and x coordinate time, ‘t’. so, the slope of the straight line which is $\tan \theta $ will give us the rate. As given, $\theta ={{\tan }^{-1}}=0.5$, this will be the rate of the reaction.
Hence, the rate of the reaction at the start is $0.5\,mol\,Lmo{{l}^{-1}}{{\min }^{-1}}$, so option D is correct.
Note:
The derivation explained is the integrated form of rate equation, rate equation states that rate is directly proportional to the concentration of reactants. Through the integration of rate equation for second order reaction, we can obtain the slope- intercept form equation, which can tell us the value of rate constant, which is the slope of the graph between concentration and time.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




