
For an endothermic reaction, energy of activation is $ {E_a} $ and enthalpy of a reaction is H (both of these in $ kJmo{l^{ - 1}} $ ). Minimum value of $ {E_a} $ will be:
A. Less than H
B. Equal to H
C. More than H
D. Equal to zero
Answer
510.6k+ views
Hint: The minimum amount of energy required by a molecule i.e., reacting species to undergo a chemical reaction is known as energy of activation for the reaction whereas enthalpy is the heat energy absorbed or released from the system during a chemical reaction performed at a constant pressure.
Complete answer:
A chemical reaction is categorized into two types on the basis of heat removal or absorption in the reaction that are exothermic reactions and endothermic reactions.
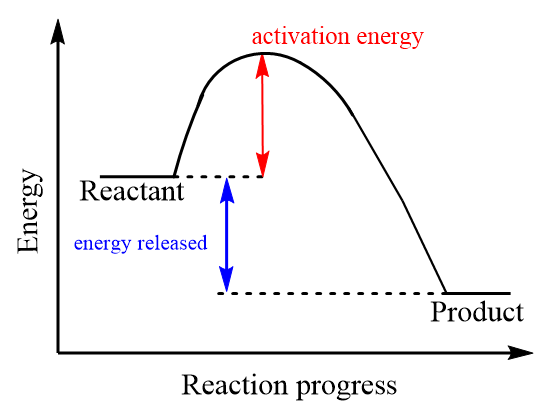
In exothermic reactions, the reactants are comparatively at higher energy than products as represented in the diagram that means the products formed in the exothermic reaction are more stable than the reactants. Therefore, overall change in enthalpy of reaction is negative i.e., heat energy is released during the reaction. The activation energy of the reaction is less than the enthalpy because relatively less amount of energy is required to convert an unstable compound to a stable compound via chemical reaction. Hence, for exothermic reactions minimum value of $ {E_a} $ is less than H. The energy profile diagram of exothermic reaction is as follows:
In endothermic reactions, the reactants are present at comparatively lower energy than the products, which means the products formed in the reaction are less stable than the reactants. Since, the reaction moves towards a more stable molecule towards a less stable molecule, the overall enthalpy change of the reaction is positive i.e., energy is absorbed from the surroundings in the form of heat. As the products are unstable, the reactants need more energy to break bonds between atoms and hence activation energy of the reaction is much larger than the enthalpy of the reaction. The energy profile diagram for an endothermic reaction is as follows:
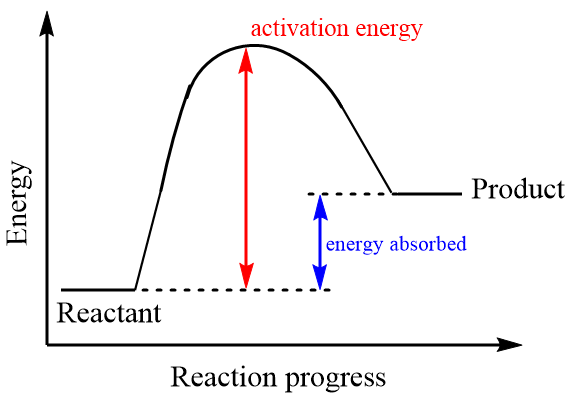
Hence, for an endothermic reaction the minimum value of $ {E_a} $ will be more than H. So, option (C) is the correct answer.
Note:
It is important to note that introducing a catalyst in a reaction can lower the activation energy of the reaction because it alters the rate of formation of product but it does not affect the enthalpy of the reaction because it solely depends on the chemical composition of reactants and products.
Complete answer:
A chemical reaction is categorized into two types on the basis of heat removal or absorption in the reaction that are exothermic reactions and endothermic reactions.
In exothermic reactions, the reactants are comparatively at higher energy than products as represented in the diagram that means the products formed in the exothermic reaction are more stable than the reactants. Therefore, overall change in enthalpy of reaction is negative i.e., heat energy is released during the reaction. The activation energy of the reaction is less than the enthalpy because relatively less amount of energy is required to convert an unstable compound to a stable compound via chemical reaction. Hence, for exothermic reactions minimum value of $ {E_a} $ is less than H. The energy profile diagram of exothermic reaction is as follows:

In endothermic reactions, the reactants are present at comparatively lower energy than the products, which means the products formed in the reaction are less stable than the reactants. Since, the reaction moves towards a more stable molecule towards a less stable molecule, the overall enthalpy change of the reaction is positive i.e., energy is absorbed from the surroundings in the form of heat. As the products are unstable, the reactants need more energy to break bonds between atoms and hence activation energy of the reaction is much larger than the enthalpy of the reaction. The energy profile diagram for an endothermic reaction is as follows:

Hence, for an endothermic reaction the minimum value of $ {E_a} $ will be more than H. So, option (C) is the correct answer.
Note:
It is important to note that introducing a catalyst in a reaction can lower the activation energy of the reaction because it alters the rate of formation of product but it does not affect the enthalpy of the reaction because it solely depends on the chemical composition of reactants and products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




