
For the complex \[{\left[ {NiC{l_4}} \right]^{2 - }}\] write (i) the IUPAC name (ii) the hybridization type (iii) the shape of the complex. (Atomic no. of Ni = 28)
Answer
582.6k+ views
Hint: Nickel (Ni) is a d- block metal. The d block elements are found in the group 3,4,5,6,7,8,9,10,11 and 12 of the periodic table. These are also known as transition metals. The d orbital is filled with an electronic shell $n - 1$ . There are a total of 40 d block elements.
Complete step by step answer:
-The d block elements are small in size and generally have high electron positive density.
They consist of $(n - 1)$ d free orbitals to accept the free electrons from the ligand and hence, form complexes easily.
-The given compound is a coordination compound. To find the IUPAC name for the given compound, we need to follow some rules:
1.Name the ions present in the compound in the order such that the name of the cation Is placed before the anion.
2.When naming within the complexion, before we write the name of the central atom, we write the name of the ligands present.
3.The names of the ligands must be written in alphabetic order.
4.In case a certain ligand is repeated, then it needs to have certain prefixes to denote the number of times it has been repeated. The prefixes mono-, di-, tri-, are used for once, twice, and thrice repetitions respectively.
5.If the ligand name is enclosed in parentheses and its number is given with the alternate prefixes bis, tris, tetrakis instead.
6.To denote the oxidation number of the central atom, the corresponding roman numeral must be included in parenthesis and be written after writing the name of the central atom.
7.When the central atom is negative, then we add the suffix ‘-ate’ to the name of the central atom.
Now considering these rules mentioned above, the IUPAC name of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrachloronickelate(II) ion.
II)
In the given metal complex, there are 4 atoms of Cl. Since Cl is a monodentate ligand, hence the charge on this ligand is (-1). From this, we can calculate the oxidation state of nickel to be 2+.
The electronic configuration of Nickel or Ni can be written as: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^8}\] . This means that the number of electrons in the last, i.e. d orbital is 8 electrons.
We know that metals with \[3{d^8}\] an electronic configuration form a square planar structure with strong field ligands and forms tetrahedral structures with weak field ligands. Also, in tetrahedral structures, the splitting of electrons happens in such a way that there remain two unpaired electrons. On the other hand, in square planar structures, all electrons are filled and have one empty subshell. This means that all charges in square planar structures are balanced and the net magnetic moment is zero.
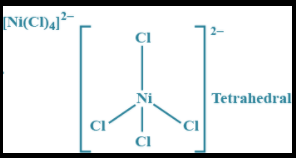
Chlorine is a weak field ligand so, the structural configuration of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrahedral. We know that the hybridization that corresponds to the tetrahedral structure is \[s{p^3}\] .
III) The shape of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrahedral.
Note:
In the case of strong field ligands, the structural configuration of \[{[NiX{}_4]^{2 - }}\] is square planar. We know that the hybridization that corresponds to the square planar structure is \[ds{p^2}\] . (X = monodentate anionic ligand). \[ds{p^2}\] type of hybridization is seen especially in the case of transition metal ions. The orbitals involved in this type of Hybridization are \[d{x^2} - {y^2}\] , s and two p. The four \[ds{p^2}\] hybrid orbitals adopt square planar geometry.
Complete step by step answer:
-The d block elements are small in size and generally have high electron positive density.
They consist of $(n - 1)$ d free orbitals to accept the free electrons from the ligand and hence, form complexes easily.
-The given compound is a coordination compound. To find the IUPAC name for the given compound, we need to follow some rules:
1.Name the ions present in the compound in the order such that the name of the cation Is placed before the anion.
2.When naming within the complexion, before we write the name of the central atom, we write the name of the ligands present.
3.The names of the ligands must be written in alphabetic order.
4.In case a certain ligand is repeated, then it needs to have certain prefixes to denote the number of times it has been repeated. The prefixes mono-, di-, tri-, are used for once, twice, and thrice repetitions respectively.
5.If the ligand name is enclosed in parentheses and its number is given with the alternate prefixes bis, tris, tetrakis instead.
6.To denote the oxidation number of the central atom, the corresponding roman numeral must be included in parenthesis and be written after writing the name of the central atom.
7.When the central atom is negative, then we add the suffix ‘-ate’ to the name of the central atom.
Now considering these rules mentioned above, the IUPAC name of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrachloronickelate(II) ion.
II)
In the given metal complex, there are 4 atoms of Cl. Since Cl is a monodentate ligand, hence the charge on this ligand is (-1). From this, we can calculate the oxidation state of nickel to be 2+.
The electronic configuration of Nickel or Ni can be written as: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^8}\] . This means that the number of electrons in the last, i.e. d orbital is 8 electrons.
We know that metals with \[3{d^8}\] an electronic configuration form a square planar structure with strong field ligands and forms tetrahedral structures with weak field ligands. Also, in tetrahedral structures, the splitting of electrons happens in such a way that there remain two unpaired electrons. On the other hand, in square planar structures, all electrons are filled and have one empty subshell. This means that all charges in square planar structures are balanced and the net magnetic moment is zero.
Chlorine is a weak field ligand so, the structural configuration of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrahedral. We know that the hybridization that corresponds to the tetrahedral structure is \[s{p^3}\] .
III) The shape of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is tetrahedral.

Note:
In the case of strong field ligands, the structural configuration of \[{[NiX{}_4]^{2 - }}\] is square planar. We know that the hybridization that corresponds to the square planar structure is \[ds{p^2}\] . (X = monodentate anionic ligand). \[ds{p^2}\] type of hybridization is seen especially in the case of transition metal ions. The orbitals involved in this type of Hybridization are \[d{x^2} - {y^2}\] , s and two p. The four \[ds{p^2}\] hybrid orbitals adopt square planar geometry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




