
For which of the pair Newland Octave rule is not applicable?
(A)- Li, Na
(B)- C, Si
(C)- Mg, Ca
(D)- Cl, Br
Answer
585.3k+ views
Hint: Newland’s Law of Octaves states that every eighth element has similar properties when the elements are arranged in the increasing order of their atomic masses.
Complete Step by step solution:
-The British chemist John Newlands in the year 1864, attempted to arrange 56 elements known at that time in the increasing order of their atomic masses.
-Newland was the first one to introduce and attempt assigning an atomic number to each element.
-Newland compared the similarity between the elements and found it similar to the musical notes Sa, Re, Ga, Ma, Pa, Dhaa, Nee, Sa. Hence he named it Newland’s Law of Octaves.
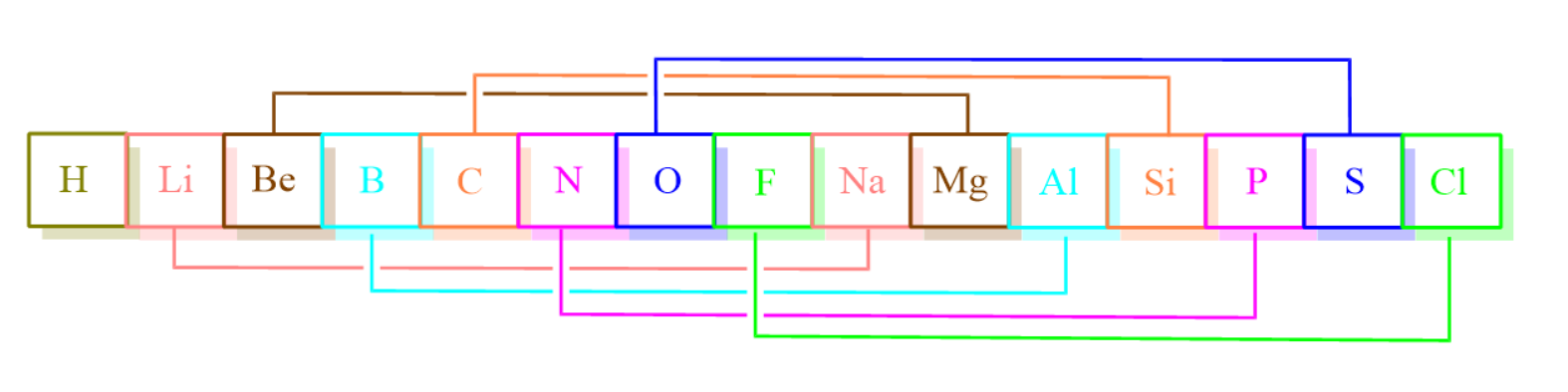
-There was a drawback of Newland’s Law of Octave that he could arrange elements only up to calcium, out of the total 56 elements known. Elements discovered after Calcium didn’t possess similar properties with every eighth element.
-The first combination of elements in option A has an atomic number as 3 and 11 respectively, hence they followed the trend of Newland’s octave.
-The second combination of elements in option B has atomic number 6 and 14 respectively, hence they also followed the trend of Newland’s octave.
-The third combination of elements in option C has atomic number 12 and 20 respectively, hence they also followed the trend of Newland’s octave.
-The fourth combination of elements in option D has atomic number 17 and 35 respectively. Cl and Br did not follow the trend of Newland’s octave as they are coming after Calcium.
So, the correct answer is option D.
Note: Following are some other limitations of Newland’s Law of Octave-
(i) At the time of Newland’s only 56 elements were discovered in nature and he assumed that no more elements will be discovered in future
(ii) Not all the elements discovered fit into the same slots in Newland’s periodic classification. For example, cobalt and nickel were placed in the same slot.
(iii) Newland adjusted two elements in two elements in the same slot which had dissimilar properties. For example, the halogens were grouped with some metals such as cobalt, nickel, and platinum.
(iv) This method of classifying elements did not leave any room for the discovery of new elements.
(v) The law after the discovery of noble gases became irrelevant.
Complete Step by step solution:
-The British chemist John Newlands in the year 1864, attempted to arrange 56 elements known at that time in the increasing order of their atomic masses.
-Newland was the first one to introduce and attempt assigning an atomic number to each element.
-Newland compared the similarity between the elements and found it similar to the musical notes Sa, Re, Ga, Ma, Pa, Dhaa, Nee, Sa. Hence he named it Newland’s Law of Octaves.

-There was a drawback of Newland’s Law of Octave that he could arrange elements only up to calcium, out of the total 56 elements known. Elements discovered after Calcium didn’t possess similar properties with every eighth element.
-The first combination of elements in option A has an atomic number as 3 and 11 respectively, hence they followed the trend of Newland’s octave.
-The second combination of elements in option B has atomic number 6 and 14 respectively, hence they also followed the trend of Newland’s octave.
-The third combination of elements in option C has atomic number 12 and 20 respectively, hence they also followed the trend of Newland’s octave.
-The fourth combination of elements in option D has atomic number 17 and 35 respectively. Cl and Br did not follow the trend of Newland’s octave as they are coming after Calcium.
So, the correct answer is option D.
Note: Following are some other limitations of Newland’s Law of Octave-
(i) At the time of Newland’s only 56 elements were discovered in nature and he assumed that no more elements will be discovered in future
(ii) Not all the elements discovered fit into the same slots in Newland’s periodic classification. For example, cobalt and nickel were placed in the same slot.
(iii) Newland adjusted two elements in two elements in the same slot which had dissimilar properties. For example, the halogens were grouped with some metals such as cobalt, nickel, and platinum.
(iv) This method of classifying elements did not leave any room for the discovery of new elements.
(v) The law after the discovery of noble gases became irrelevant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




