
What is the formal charge on Cl in the following Lewis structure?
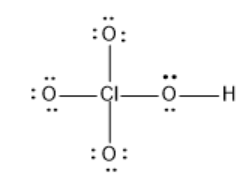
(A) 0
(B) +3
(C) +5
(D) +7

Answer
503.4k+ views
Hint :The given Structure is $ HClO_4 $ that is Perchloric acid. The Central Atom Chlorine is bonded to 4 oxygen atoms. Each bond between oxygen and chlorine depicts the presence of two electrons. The two electrons which occur in pairs in the Lewis structure are the non-bonding electrons.
Complete Step By Step Answer:
$ HClO_4 $ is a polyatomic molecule with 34 valence electrons.
The formal charge on a polyatomic ion or molecule is the difference between the number of valence electrons of the element minus the sum of the number of nonbonding electrons plus the number of bonding electrons.
Chlorine has the atomic number 17. It has 7 valence electrons in the elemental state. The outer shell of chlorine is filled with 8 electrons and has a stable configuration.
Oxygen has valency 6 in the elemental state. The three oxygen atoms have 6 unpaired electrons on them. The oxygen bonded to hydrogen has 4 electrons.
We have to calculate the formal charge on Chlorine.
The formula for finding the formal charge is-
$ \Rightarrow Formal\text{ }Charge\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }electrons\text{ }present\text{ }in\text{ }the\text{ }Lewis\text{ }structure \right] $
$ \Rightarrow F.C.\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }number\text{ }of\text{ }non-bonding\text{ }electrons\text{ + }\frac{1}{2}\left( Total\text{ }of\text{ }bonding\text{ }electrons \right) \right] $
Half the total number of bonding electrons is the number of bonds formed by the atom.
$ \Rightarrow Formal\text{ }Charge\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }number\text{ }of\text{ }non-bonding\text{ }electrons\text{ + }\left( Total\text{ }number\text{ }of\text{ }bonds\text{ }formed \right) \right] $
For chlorine,
Total valence electrons= 7
Total number of nonbonding electrons= 0
Total number of bonds formed= 4
Substituting the above numbers in the formula,
F.C. on Chlorine $= \left[ 7-\left( 0+4 \right) \right] $
$=\text{ }\left[ 7-4 \right] $
$=\text{ }3 $
Final answer: Correct answer is $ Option\text{ }B\text{ }\Rightarrow +3 $ .
Note :
In the given structure of $ HClO_4 $ it doesn’t show the presence of electrons on Chlorine, but instead has bonds. The bond depicts the presence of one electron pair shared between the oxygen atom and the chlorine atom. The Lewis structure of any molecule shows the outer shell configuration of the atom.
Complete Step By Step Answer:
$ HClO_4 $ is a polyatomic molecule with 34 valence electrons.
The formal charge on a polyatomic ion or molecule is the difference between the number of valence electrons of the element minus the sum of the number of nonbonding electrons plus the number of bonding electrons.
Chlorine has the atomic number 17. It has 7 valence electrons in the elemental state. The outer shell of chlorine is filled with 8 electrons and has a stable configuration.
Oxygen has valency 6 in the elemental state. The three oxygen atoms have 6 unpaired electrons on them. The oxygen bonded to hydrogen has 4 electrons.
We have to calculate the formal charge on Chlorine.
The formula for finding the formal charge is-
$ \Rightarrow Formal\text{ }Charge\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }electrons\text{ }present\text{ }in\text{ }the\text{ }Lewis\text{ }structure \right] $
$ \Rightarrow F.C.\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }number\text{ }of\text{ }non-bonding\text{ }electrons\text{ + }\frac{1}{2}\left( Total\text{ }of\text{ }bonding\text{ }electrons \right) \right] $
Half the total number of bonding electrons is the number of bonds formed by the atom.
$ \Rightarrow Formal\text{ }Charge\text{ }=\text{ }\left[ Total\text{ }valence\text{ }electrons\text{ }in\text{ }free\text{ }state \right]\text{ }\text{ }\left[ Total\text{ }number\text{ }of\text{ }non-bonding\text{ }electrons\text{ + }\left( Total\text{ }number\text{ }of\text{ }bonds\text{ }formed \right) \right] $
For chlorine,
Total valence electrons= 7
Total number of nonbonding electrons= 0
Total number of bonds formed= 4
Substituting the above numbers in the formula,
F.C. on Chlorine $= \left[ 7-\left( 0+4 \right) \right] $
$=\text{ }\left[ 7-4 \right] $
$=\text{ }3 $
Final answer: Correct answer is $ Option\text{ }B\text{ }\Rightarrow +3 $ .
Note :
In the given structure of $ HClO_4 $ it doesn’t show the presence of electrons on Chlorine, but instead has bonds. The bond depicts the presence of one electron pair shared between the oxygen atom and the chlorine atom. The Lewis structure of any molecule shows the outer shell configuration of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




