
Formaldehyde at room temperature is:
A.Liquid
B.Gas
C.Solid
D.None of the above
Answer
587.4k+ views
Hint: Formaldehyde is a colorless, strong smelling gas used in making building materials and many household products. It is a naturally occurring organic compound with chemical formula. It does not accumulate in the environment because it is broken down within a few hours by sunlight or by the bacteria present in the soil or water.
Complete step by step answer:
Formaldehyde is the simplest aldehyde made of hydrogen, carbon and oxygen. It is one of a large family of chemicals known as volatile organic compounds which evaporates and becomes gaseous at room temperature.
Further, its chemical formula is HCHO and the structure is as shown:
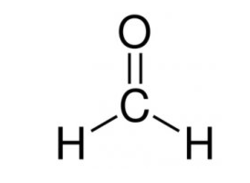
It reacts with sodium hydroxide and forms sodium formate and methanol. The chemical equation is given below:
$2HCHO + NaOH \to HCOONa + C{H_3}OH$
Further, it reacts with ammonia and forms formamidine and water. The equation is as shown:
$6HCHO + 4N{H_3} \to (C{H_2})_6{N_4} + 6{H_2}O$
Hence, option B is correct.
Additional information:
Upon the addition of hydrogen, the formic acid is converted to formaldehyde. So, formaldehyde is more easily oxidized into formic acid by ambient oxygen. Moreover, formic acid is miscible with water and many other organic solvents as well.
Note:
Formaldehyde is used in the production of resins, principally urea formaldehyde and phenol-formaldehyde are used to make cores and moulds for foundries. Moreover, it has a long history of safe use in producing some bacterial and viral vaccines and because of its antibacterial properties, it is widely used as a preservative.
Complete step by step answer:
Formaldehyde is the simplest aldehyde made of hydrogen, carbon and oxygen. It is one of a large family of chemicals known as volatile organic compounds which evaporates and becomes gaseous at room temperature.
Further, its chemical formula is HCHO and the structure is as shown:

It reacts with sodium hydroxide and forms sodium formate and methanol. The chemical equation is given below:
$2HCHO + NaOH \to HCOONa + C{H_3}OH$
Further, it reacts with ammonia and forms formamidine and water. The equation is as shown:
$6HCHO + 4N{H_3} \to (C{H_2})_6{N_4} + 6{H_2}O$
Hence, option B is correct.
Additional information:
Upon the addition of hydrogen, the formic acid is converted to formaldehyde. So, formaldehyde is more easily oxidized into formic acid by ambient oxygen. Moreover, formic acid is miscible with water and many other organic solvents as well.
Note:
Formaldehyde is used in the production of resins, principally urea formaldehyde and phenol-formaldehyde are used to make cores and moulds for foundries. Moreover, it has a long history of safe use in producing some bacterial and viral vaccines and because of its antibacterial properties, it is widely used as a preservative.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




