
What is formed if a mixture of powdered iron and sulphur is heated in a test tube?
A.An element
B.A mixture
C.A compound
D.None of the above
Answer
580.2k+ views
Hint: A mixture is a combination of matter such that the components used in the formation of the mixture can be separated again.
While a compound is defined as the substance result of the chemical reaction between components resulting in the formation of a new substance.
Complete step by step answer:
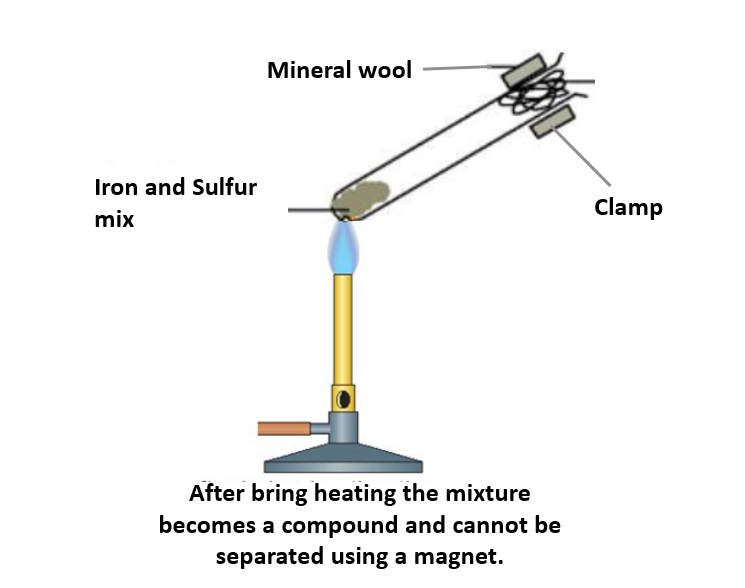
Take some iron filings and sulphur in powder form. Stir the two elements to form a mixture. The two components of the mixture can be separated with the help of a magnet.
This reaction is an exothermic reaction of two elements, iron and sulphur, to form the compound named iron sulphide. The two solids can be mixed and heated in a test-tube (or ignition tube). The chemical reaction can be used to illustrate different elements, mixtures and compounds.
A mixture is made by combining matter in a way where the components can be separated again. A compound formed from a chemical reaction between components is a new substance. For example, when you make a mixture from iron filings with sulphur, you just need a magnet to separate the iron from the sulphur. On the other hand, when you heat the iron and sulphur, iron sulphide is formed which is a compound. \[Fe + S \to FeS\]
So, the correct answer is Option B.
Note:
On heating the reaction mixture, the sulphur melts and reacts with the iron exothermically to form iron (II) sulphide. The mineral wool plug in the mouth of the test tube prevents sulphur vapour from escaping and possibly catching fire. If, even after all precautions taken, the sulphur vapour does ignite, students should be trained to extinguish the fire by placing a damp rag firmly over the mouth of the tube.
Exothermic reactions are defined as reactions or processes that release energy which is usually in the form of heat or light. In these reactions, energy is released during the reaction because the total energy of the products contained is less than the total energy of the reactants released.
While a compound is defined as the substance result of the chemical reaction between components resulting in the formation of a new substance.
Complete step by step answer:
Take some iron filings and sulphur in powder form. Stir the two elements to form a mixture. The two components of the mixture can be separated with the help of a magnet.
This reaction is an exothermic reaction of two elements, iron and sulphur, to form the compound named iron sulphide. The two solids can be mixed and heated in a test-tube (or ignition tube). The chemical reaction can be used to illustrate different elements, mixtures and compounds.
A mixture is made by combining matter in a way where the components can be separated again. A compound formed from a chemical reaction between components is a new substance. For example, when you make a mixture from iron filings with sulphur, you just need a magnet to separate the iron from the sulphur. On the other hand, when you heat the iron and sulphur, iron sulphide is formed which is a compound. \[Fe + S \to FeS\]

So, the correct answer is Option B.
Note:
On heating the reaction mixture, the sulphur melts and reacts with the iron exothermically to form iron (II) sulphide. The mineral wool plug in the mouth of the test tube prevents sulphur vapour from escaping and possibly catching fire. If, even after all precautions taken, the sulphur vapour does ignite, students should be trained to extinguish the fire by placing a damp rag firmly over the mouth of the tube.
Exothermic reactions are defined as reactions or processes that release energy which is usually in the form of heat or light. In these reactions, energy is released during the reaction because the total energy of the products contained is less than the total energy of the reactants released.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




