
What is the formula for the compound dinitrogen pentoxide?
Answer
481.5k+ views
Hint: To answer this we have to analyze the given name of the compound, like the numbers of respective atoms there. We can write the formula by using the prefix such as di which means $ 2 $ and pent which means $ 5 $ .
Complete Step By Step Answer:
Dinitrogen pentoxide is a compound, alternatively termed as nitrogen pentoxide or nitric anhydride which consists of only nitrogen and oxygen. As by the name we can understand that a Dinitrogen pentoxide molecule has two atoms of nitrogen and five atoms of oxygen atoms. Thus the chemical formula of this compound is $ {N_2}{O_5} $ .
The anhydride form of nitric acid is dinitrogen pentoxide which is the colourless crystal. Its melting point is $ {30^ \circ }C $ . Dinitrogen pentoxide is used as the oxidiser in many chemical reactions, unlike that unstable oxide of nitrogen. It can be used as a nitrating agent. However, it is now replaced by nitronium tetrafluoroborate .
Dinitrogen pentoxide is made of $ 2 $ nitrogen atoms and $ 5 $ oxygen atoms so if we want to draw the Lewis Dot Structure of $ {N_2}{O_5} $ , you have to know that:
Nitrogen has five valence electrons and oxygen has six valence electrons thus, the structure of dinitrogen pentoxide which is $ {N_2}{O_5} $ , is:
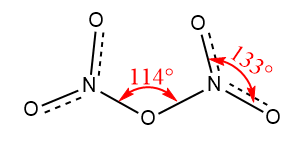
Dinitrogen pentoxide is the compound which contains nitrogen and oxygen elements. It is a binary compound which means those compounds which contain only two elements. It exists in the form of solid at room temperature but becomes colourless gas when temperature is raised slightly above the room temperature.
Note:
As the nitrogen contains five valence electrons; it is able to form different types of oxides which are di-nitrogen oxygen, nitrogen monoxide, dinitrogen trioxide, and nitrogen dioxide and nitrogen pentoxide.
Complete Step By Step Answer:
Dinitrogen pentoxide is a compound, alternatively termed as nitrogen pentoxide or nitric anhydride which consists of only nitrogen and oxygen. As by the name we can understand that a Dinitrogen pentoxide molecule has two atoms of nitrogen and five atoms of oxygen atoms. Thus the chemical formula of this compound is $ {N_2}{O_5} $ .
The anhydride form of nitric acid is dinitrogen pentoxide which is the colourless crystal. Its melting point is $ {30^ \circ }C $ . Dinitrogen pentoxide is used as the oxidiser in many chemical reactions, unlike that unstable oxide of nitrogen. It can be used as a nitrating agent. However, it is now replaced by nitronium tetrafluoroborate .
Dinitrogen pentoxide is made of $ 2 $ nitrogen atoms and $ 5 $ oxygen atoms so if we want to draw the Lewis Dot Structure of $ {N_2}{O_5} $ , you have to know that:
Nitrogen has five valence electrons and oxygen has six valence electrons thus, the structure of dinitrogen pentoxide which is $ {N_2}{O_5} $ , is:

Dinitrogen pentoxide is the compound which contains nitrogen and oxygen elements. It is a binary compound which means those compounds which contain only two elements. It exists in the form of solid at room temperature but becomes colourless gas when temperature is raised slightly above the room temperature.
Note:
As the nitrogen contains five valence electrons; it is able to form different types of oxides which are di-nitrogen oxygen, nitrogen monoxide, dinitrogen trioxide, and nitrogen dioxide and nitrogen pentoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




