
What is the formula of aluminium phosphate?
Answer
495.6k+ views
Hint: Molecular formulas of any given compound provide the exact number of atoms of different elements which are present in one mole of compound. Molecular formulas help to determine the chemical and physical nature of compounds depending upon the nature of its constituents.
Complete answer:
There are some basic rules to write the formula of any compound-
First of all, we have to write the symbols of all the atoms which are present in molecules.
Symbols of metal atoms should always be mentioned before the symbol of non-metal.
If ions are present in the compound then mention the symbol of ion in brackets.
Now mention valency of all the atoms below their symbol.
Finally, we have to cross matched the valence of atoms to find the chemical formula.
In the case of aluminium phosphate, as we know aluminium is metal so it is mentioned before the phosphate. Atomic number of aluminium is $13$ and the electronic configuration is $\left[ {Ne} \right]3{s^2}3{p^1}$. Hence, there are three electrons in the outermost shell of aluminium. When aluminium loses its three electrons it is converted into $\left( {A{l^{ + 3}}} \right)$ ions.
Similarly, the atomic number of phosphorus is $15$ and electronic configuration is $\left[ {Ne} \right]3{s^2}3{p^3}$. Hence, there are five electrons present in the outermost shell. When it gains two electrons to complete its outermost shell it will be converted into ${\left( {P{O_4}} \right)^{ - 3}}$.
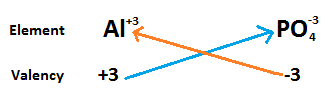
On combining they form $3\left( {A{l^{ + 3}}} \right)3\left( {P{O_4}^{ - 3}} \right)$. As we see that positive and negative charges are present in molecules of the same magnitude so they cancel each other to form neutral compounds.
$ \Rightarrow $ So, the formula of aluminium phosphate $\left( {AlP{O_4}} \right)$.
Note:
Empirical formula is another way to express the formula of any given compound in the simple whole number ratio of atoms of different elements present in one molecule of compound. Molecular formula is equal to the product of empirical formula and n-factor of compound which is a whole integer.
Complete answer:
There are some basic rules to write the formula of any compound-
First of all, we have to write the symbols of all the atoms which are present in molecules.
Symbols of metal atoms should always be mentioned before the symbol of non-metal.
If ions are present in the compound then mention the symbol of ion in brackets.
Now mention valency of all the atoms below their symbol.
Finally, we have to cross matched the valence of atoms to find the chemical formula.
In the case of aluminium phosphate, as we know aluminium is metal so it is mentioned before the phosphate. Atomic number of aluminium is $13$ and the electronic configuration is $\left[ {Ne} \right]3{s^2}3{p^1}$. Hence, there are three electrons in the outermost shell of aluminium. When aluminium loses its three electrons it is converted into $\left( {A{l^{ + 3}}} \right)$ ions.
Similarly, the atomic number of phosphorus is $15$ and electronic configuration is $\left[ {Ne} \right]3{s^2}3{p^3}$. Hence, there are five electrons present in the outermost shell. When it gains two electrons to complete its outermost shell it will be converted into ${\left( {P{O_4}} \right)^{ - 3}}$.

On combining they form $3\left( {A{l^{ + 3}}} \right)3\left( {P{O_4}^{ - 3}} \right)$. As we see that positive and negative charges are present in molecules of the same magnitude so they cancel each other to form neutral compounds.
$ \Rightarrow $ So, the formula of aluminium phosphate $\left( {AlP{O_4}} \right)$.
Note:
Empirical formula is another way to express the formula of any given compound in the simple whole number ratio of atoms of different elements present in one molecule of compound. Molecular formula is equal to the product of empirical formula and n-factor of compound which is a whole integer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




