
Formula of Aluminium sulphide:
$
{\text{A}}{\text{. A}}{{\text{l}}_2}{\text{S}}{{\text{O}}_4} \\
{\text{B}}{\text{. A}}{{\text{l}}_2}{\text{S}}{{\text{O}}_3} \\
{\text{C}}{\text{. A}}{{\text{l}}_2}{\text{S}} \\
{\text{D}}{\text{. A}}{{\text{l}}_2}{{\text{S}}_3} \\
$
Answer
607.8k+ views
Hint:- In this question first we know about the elements involved in the formula of “Aluminium Sulphide” then we have to apply the criss-cross method to determine the formula of “Aluminium Sulphide”.
Step-By-Step solution:
Aluminium is a post- transition metal with atomic number 13. Sulphur is a non-metal with atomic number 16. Aluminium Sulphide is produced by reacting aluminium and sulphur, which is an exothermic reaction.
Aluminium sulphide is an ionic compound, which is a combination of positive and negative ions.
An ion is an atom or molecule that has a charge because it has too many or too few electrons. Ions are either monoatomic (consisting of one atom) or polyatomic (consisting of more than one atom). Many of the most common ionic compounds have a positive ion consisting of one metal atom and a negative ion that's polyatomic. It's not a rule, but it's a helpful pattern.
The positive ion in aluminium sulphide is aluminium(${\text{A}}{{\text{l}}^{3 + }}$) and the sulphide is the negative ion(${{\text{S}}^{2 - }}$).
All ionic compounds have to be electrically neutral, so we have to put the Aluminium and Sulphide ions together in the correct ratio so their electrical charges cancel. There is a trick to determine that ratio; it's called ''the criss-cross'' method.
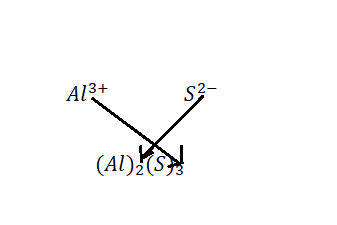
Therefore, the formula of Aluminium sulphide is ${\text{A}}{{\text{l}}_2}{{\text{S}}_3}$.
Hence, option D. is correct.
Note:- Whenever you get this type of question the key concept to solve this is to learn about the different elements in a compound and charges on them after attaining the completion of octet like in this case charge on Aluminium ion is +3 and charge on sulphide ion is -2.
Step-By-Step solution:
Aluminium is a post- transition metal with atomic number 13. Sulphur is a non-metal with atomic number 16. Aluminium Sulphide is produced by reacting aluminium and sulphur, which is an exothermic reaction.
Aluminium sulphide is an ionic compound, which is a combination of positive and negative ions.
An ion is an atom or molecule that has a charge because it has too many or too few electrons. Ions are either monoatomic (consisting of one atom) or polyatomic (consisting of more than one atom). Many of the most common ionic compounds have a positive ion consisting of one metal atom and a negative ion that's polyatomic. It's not a rule, but it's a helpful pattern.
The positive ion in aluminium sulphide is aluminium(${\text{A}}{{\text{l}}^{3 + }}$) and the sulphide is the negative ion(${{\text{S}}^{2 - }}$).
All ionic compounds have to be electrically neutral, so we have to put the Aluminium and Sulphide ions together in the correct ratio so their electrical charges cancel. There is a trick to determine that ratio; it's called ''the criss-cross'' method.
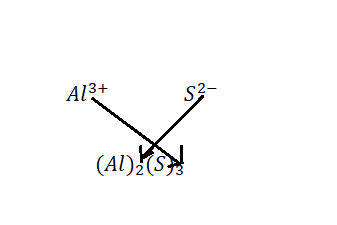
Therefore, the formula of Aluminium sulphide is ${\text{A}}{{\text{l}}_2}{{\text{S}}_3}$.
Hence, option D. is correct.
Note:- Whenever you get this type of question the key concept to solve this is to learn about the different elements in a compound and charges on them after attaining the completion of octet like in this case charge on Aluminium ion is +3 and charge on sulphide ion is -2.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




