
From recorder data:
1. Weight of U-tube $ 20.36g. $
2. Weight of U-tube and calcium chloride before $ 39.22g. $
3. Weight of U-tube and calcium chloride after $ 57.32g. $
4. Weight of boat and contents (copper oxide) before $ 30.23g $
5. Weight of boat and contents after $ 14.23g $
6. Weight of boat $ 5.00g $
7. What is the reaction for the first $ CaC{{l}_{2}} $ drying tube?
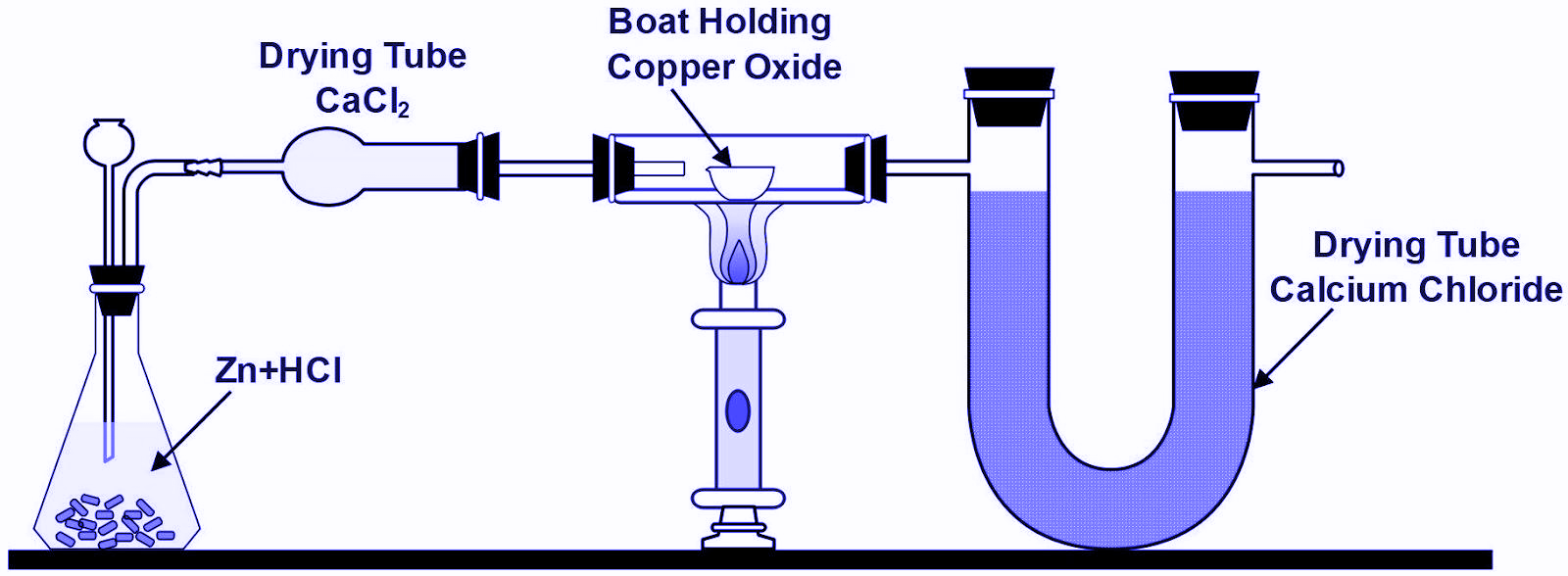
(A) Generate Water.
(B) Absorb Hydrogen.
(C) Absorb water that evaporates from the flask.
(D) Decompose the water from the flask.

Answer
510.6k+ views
Hint: We know that the calcium chloride is soluble in water. It can be made by neutralizing hydrochloric acid with calcium hydroxide. It is commonly encountered as a hydrated solid. For de-icing and dust control these compounds are used.
Complete answer:
If we see how hydrogen chloride gas is prepared, we will see that the gas is usually passed through a guard tube (also known as a drying tube) containing anhydrous calcium chloride. The reason behind why anhydrous calcium chloride is used in guard tubes is because it is considered as a very good drying agent. If we see what a drying agent is: we will get to know that drying agents acquire water of hydration when they are exposed to wet solutions or moist air. If we see overall properties of anhydrous calcium chloride we will conclude that it is the best example or best used as a dehydrating or drying agent which absorbs the moisture of water content from the gas hence making it dry. Overall if we see and consider all the above facts we will see that the main role of anhydrous calcium chloride is to absorb moisture from the gas.
The first $ CaC{{l}_{2}} $ drying tube absorbs water that evaporates from the flask. $ Zn $ reacts with $ HCl $ to produce hydrogen gas. The hydrogen gas contains moisture. When it passes through the first $ CaC{{l}_{2}} $ drying tube, the moisture is absorbed by $ CaC{{l}_{2}} $ which is a drying agent. The hydrogen gas coming out of the first $ CaC{{l}_{2}} $ drying tube is dry and free of moisture.
Therefore, the correct answer is option C.
Note:
Remember that the main property that we should remember is that anhydrous calcium chloride is considered as a very good drying agent as it absorbs moisture. Anhydrous calcium chloride has a very good tendency of holding the water molecules and hence it is used as a desiccant in most of the experiments in the guard tube.
Complete answer:
If we see how hydrogen chloride gas is prepared, we will see that the gas is usually passed through a guard tube (also known as a drying tube) containing anhydrous calcium chloride. The reason behind why anhydrous calcium chloride is used in guard tubes is because it is considered as a very good drying agent. If we see what a drying agent is: we will get to know that drying agents acquire water of hydration when they are exposed to wet solutions or moist air. If we see overall properties of anhydrous calcium chloride we will conclude that it is the best example or best used as a dehydrating or drying agent which absorbs the moisture of water content from the gas hence making it dry. Overall if we see and consider all the above facts we will see that the main role of anhydrous calcium chloride is to absorb moisture from the gas.
The first $ CaC{{l}_{2}} $ drying tube absorbs water that evaporates from the flask. $ Zn $ reacts with $ HCl $ to produce hydrogen gas. The hydrogen gas contains moisture. When it passes through the first $ CaC{{l}_{2}} $ drying tube, the moisture is absorbed by $ CaC{{l}_{2}} $ which is a drying agent. The hydrogen gas coming out of the first $ CaC{{l}_{2}} $ drying tube is dry and free of moisture.
Therefore, the correct answer is option C.
Note:
Remember that the main property that we should remember is that anhydrous calcium chloride is considered as a very good drying agent as it absorbs moisture. Anhydrous calcium chloride has a very good tendency of holding the water molecules and hence it is used as a desiccant in most of the experiments in the guard tube.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




