
What functional group is in ethyl butyrate?
Answer
524.4k+ views
Hint : We know that we know that the ester is a chemical compound which is derived from an acid, in which an \[OH\] group is replaced by \[OR\]group. So, the structure of ether is\[RCOOR\]. Here, R represents the alkyl groups.
Complete Step By Step Answer:
Let’s discuss the naming of an ester. The naming of ester is done as if the alkyl chain from the alcohol is a substituent. Then, we do not need to assign numbers to the alkyl chain. Then, we have to name the parent chain from the carboxylic acid part of ester. Then, the ‘e’ of the parent should be replaced with ‘oate’. Then, the \[R\]group bonded to the \[O\]atom is named as substituent (prefix).
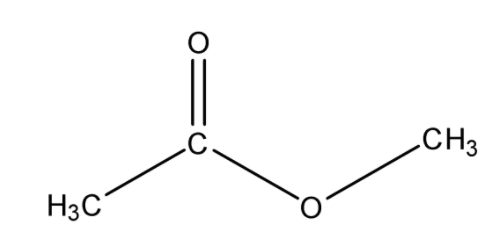
Ethyl butyrate is another name for ethyl butanoate. Some common names and their corresponding IUPAC equivalents are:
formate $=$ methanoate
acetate $=$ ethanoate
propionate $=$ propanoate
butyrate $=$ butanoate
valerate $=$ pentanoate
An alkanoate is a salt or ester derivative of a carboxylic acid group (such as sodium acetate/ethanoate), so ethyl butanoate is the ester derivative of butanoic acid. It forms from an esterification reaction of ethanol (ethyl alcohol) with butanoic acid on high heat in the presence of an acid catalyst (such as \[{{H}_{2}}S{{O}_{4}}\]) to remove the \[OH\] from the alcohol and the \[H\] from the carboxylic acid.
Note :
Remember that it is to be noted that when carboxylic acid undergoes reaction with alcohol in the presence of an acid catalyst, ester formation takes place. The acid catalyst that is commonly used in this reaction is concentrated sulphuric acid .
Complete Step By Step Answer:
Let’s discuss the naming of an ester. The naming of ester is done as if the alkyl chain from the alcohol is a substituent. Then, we do not need to assign numbers to the alkyl chain. Then, we have to name the parent chain from the carboxylic acid part of ester. Then, the ‘e’ of the parent should be replaced with ‘oate’. Then, the \[R\]group bonded to the \[O\]atom is named as substituent (prefix).
Ethyl butyrate is another name for ethyl butanoate. Some common names and their corresponding IUPAC equivalents are:
formate $=$ methanoate
acetate $=$ ethanoate
propionate $=$ propanoate
butyrate $=$ butanoate
valerate $=$ pentanoate
An alkanoate is a salt or ester derivative of a carboxylic acid group (such as sodium acetate/ethanoate), so ethyl butanoate is the ester derivative of butanoic acid. It forms from an esterification reaction of ethanol (ethyl alcohol) with butanoic acid on high heat in the presence of an acid catalyst (such as \[{{H}_{2}}S{{O}_{4}}\]) to remove the \[OH\] from the alcohol and the \[H\] from the carboxylic acid.

Note :
Remember that it is to be noted that when carboxylic acid undergoes reaction with alcohol in the presence of an acid catalyst, ester formation takes place. The acid catalyst that is commonly used in this reaction is concentrated sulphuric acid .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




