
What functional groups are in Caffeine ?
Answer
521.1k+ views
Hint :We know that to solve the given problem, we should have the basic knowledge of Caffeine and functional groups. Organic Chemistry plays an important role in the manufacturing of many daily use products, Caffeine is one of them. It belongs to the methylxanthine class.
Complete Step By Step Answer:
Functional groups are specific groups of atoms or bonds in a compound which is responsible for the characteristic chemical reaction of that compound. Same functional group will behave in similar fashion, regardless of the compound of which it is part of. It is a common psychoactive drug which increases and alters the psychological changes in our body. It is made up of organic compounds. So, it definitely contains some functional groups.
Step-1: We should know that functional groups are always specific containing the precise atoms in the similar molecular formula always. They have some specific chemical and physical properties that do not change.
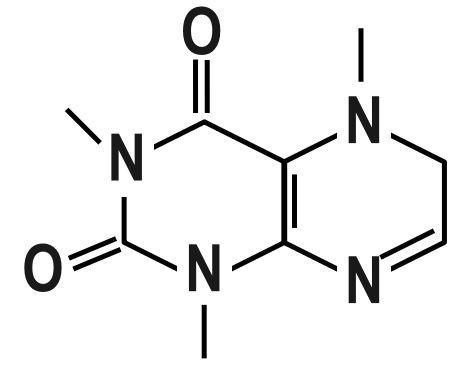
Step-2: There are many functional groups which should be remembered correctly with their formulas. Some of them are Carboxylic acid ( $ -COOH $ ), Amide ( $ -CON{{H}_{2}} $ ), Ketone ( $ -CO $ ), Aldehyde ( $ -CHO $ ), Alcohol ( $ -OH $ ), Amine ( $ -N{{H}_{2}} $ ) and many more.
Step-3: On studying the structure of Caffeine properly, we can say that they have amide and amine as functional groups. The structure contains $ 1 $ amine and $ 2 $ amide groups. It looked carefully and precisely, an imine group ( $ -C=N $ ) is also present. The general groups like methyl ( $ -C{{H}_{3}} $ ) and alkene ( $ -C=C $ ) groups are also found in the compound.
Note :
Remember that the chemical formula for a functional group or any group changes while in a straight line and in a cyclic structure. So, it should be reminded carefully. An organic compound can contain more than one functional group. To identify the functional group, you must know their formula. For example, if a hydroxyl group is present then its formula is $ -OH. $ For the acidic group, the formula is $ -COOH.~ $
Complete Step By Step Answer:
Functional groups are specific groups of atoms or bonds in a compound which is responsible for the characteristic chemical reaction of that compound. Same functional group will behave in similar fashion, regardless of the compound of which it is part of. It is a common psychoactive drug which increases and alters the psychological changes in our body. It is made up of organic compounds. So, it definitely contains some functional groups.
Step-1: We should know that functional groups are always specific containing the precise atoms in the similar molecular formula always. They have some specific chemical and physical properties that do not change.

Step-2: There are many functional groups which should be remembered correctly with their formulas. Some of them are Carboxylic acid ( $ -COOH $ ), Amide ( $ -CON{{H}_{2}} $ ), Ketone ( $ -CO $ ), Aldehyde ( $ -CHO $ ), Alcohol ( $ -OH $ ), Amine ( $ -N{{H}_{2}} $ ) and many more.
Step-3: On studying the structure of Caffeine properly, we can say that they have amide and amine as functional groups. The structure contains $ 1 $ amine and $ 2 $ amide groups. It looked carefully and precisely, an imine group ( $ -C=N $ ) is also present. The general groups like methyl ( $ -C{{H}_{3}} $ ) and alkene ( $ -C=C $ ) groups are also found in the compound.
Note :
Remember that the chemical formula for a functional group or any group changes while in a straight line and in a cyclic structure. So, it should be reminded carefully. An organic compound can contain more than one functional group. To identify the functional group, you must know their formula. For example, if a hydroxyl group is present then its formula is $ -OH. $ For the acidic group, the formula is $ -COOH.~ $
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




