
Give a simple chemical test to distinguish between Benzaldehyde and Ethanal.
Answer
564.6k+ views
Hint: Both the compounds have the Aldehyde group$ - CHO$ in their structure and they are the part of the homologous series of the aldehydes. Where the difference arises because of the change in the chemical properties only.
Complete step by step answer:
-Benzaldehyde is an organic compound having the molecular formula of ${C_6}{H_5}CHO$ a benzene ring is present in the structure of the compound. It is the industrially most used and the simplest aromatic aldehyde. It is colourless liquid with the characteristic almond odour.
-Ethanal is commonly known as acetaldehyde having the molecular formula $C{H_3}CHO$, it occurs widely in nature and is widely used in the industries as well. It occurs naturally in coffee, bread and ripened fruits as well.
-Iodoform test is used to distinguish between these two aldehydes and the test is used to detect the presence of the carbonyl compounds with the structure $R - CHO - C{H_3}$ in the given compound.
Mechanism of the Iodoform test:
-In this test the iodine reacts with the base and the methyl ketone and gives the yellow precipitate having the characteristic antiseptic smell. In the test when the base such as sodium hydroxide is added to along with the iodine in the reaction, if the compound contains a methyl ketone or secondary alcohol with the methyl group in the alpha position a pale yellow precipitate is formed, which is formed in the case of the ethanal where methyl group CH3 is present thus it gives the positive Iodoform test while benzaldehyde does not gives the positive Iodoform test.
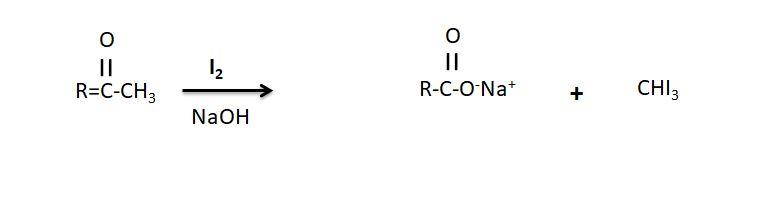
Note:
The reliability of this reaction is such that it is frequently used to determine the presence of the methyl ketone in the given unknown compound, and is used to find the alpha positive position in the secondary alcohols.
Complete step by step answer:
-Benzaldehyde is an organic compound having the molecular formula of ${C_6}{H_5}CHO$ a benzene ring is present in the structure of the compound. It is the industrially most used and the simplest aromatic aldehyde. It is colourless liquid with the characteristic almond odour.
-Ethanal is commonly known as acetaldehyde having the molecular formula $C{H_3}CHO$, it occurs widely in nature and is widely used in the industries as well. It occurs naturally in coffee, bread and ripened fruits as well.
-Iodoform test is used to distinguish between these two aldehydes and the test is used to detect the presence of the carbonyl compounds with the structure $R - CHO - C{H_3}$ in the given compound.
Mechanism of the Iodoform test:
-In this test the iodine reacts with the base and the methyl ketone and gives the yellow precipitate having the characteristic antiseptic smell. In the test when the base such as sodium hydroxide is added to along with the iodine in the reaction, if the compound contains a methyl ketone or secondary alcohol with the methyl group in the alpha position a pale yellow precipitate is formed, which is formed in the case of the ethanal where methyl group CH3 is present thus it gives the positive Iodoform test while benzaldehyde does not gives the positive Iodoform test.
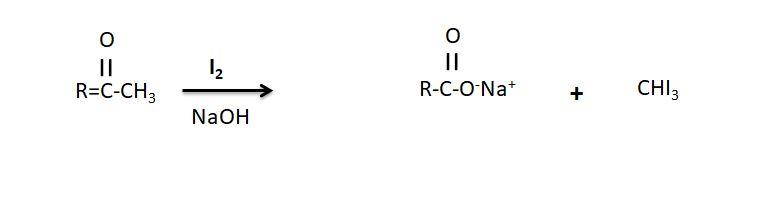
Note:
The reliability of this reaction is such that it is frequently used to determine the presence of the methyl ketone in the given unknown compound, and is used to find the alpha positive position in the secondary alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




