
Give a simple test to differentiate cyclohexane and cyclohexene.
A. \[B{r_2}{H_2}O\]
B. Bayer’s reagent
C. Tollen’s reagent
D. Both (A) and (B)
Answer
591.3k+ views
Hint: To differentiate between cyclohexane and cyclohexene first we need to understand the basic difference between both of them. So, let’s first draw the structure of both cyclic alkane cyclohexane and cyclic alkene cyclohexene organic compounds.
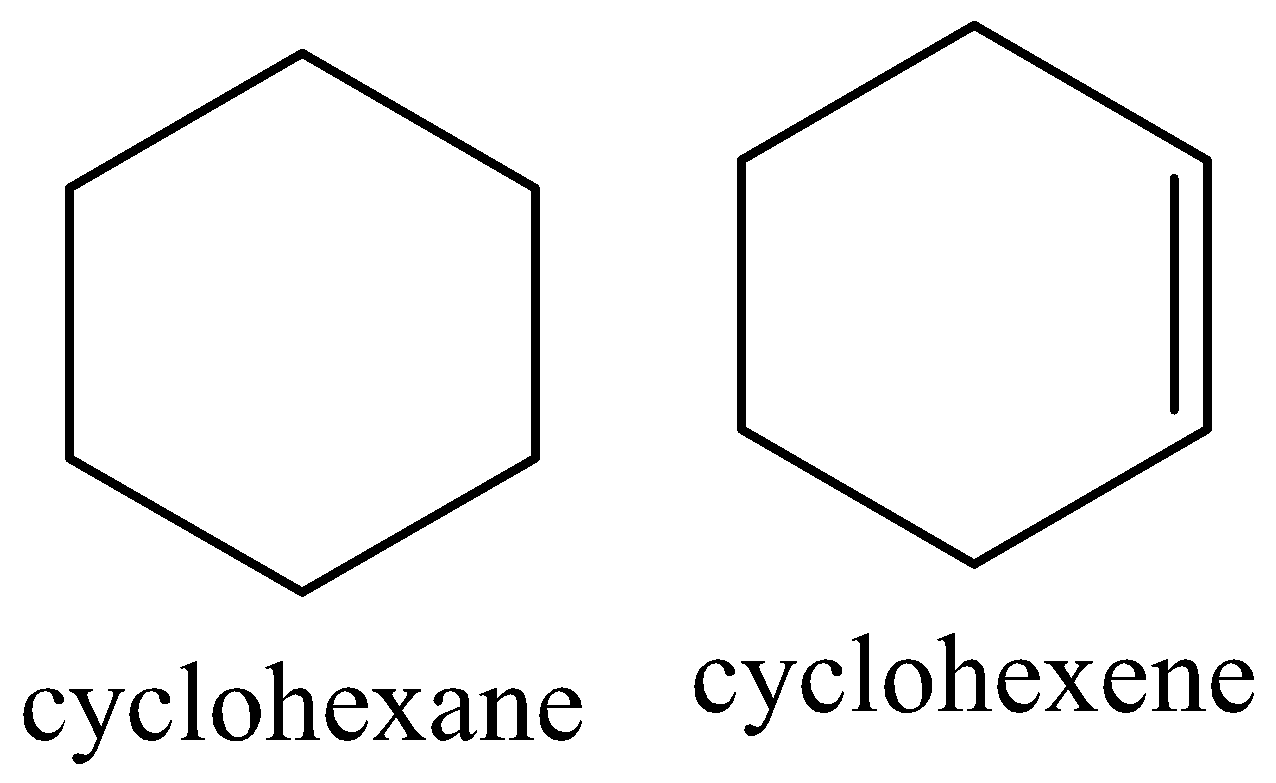
Complete step by step answer:
Cyclic cyclohexane is ${1^ \circ }$ saturated hydrocarbon and cyclic cyclohexene is ${2^ \circ }$ unsaturated hydrocarbon as cyclohexane has one ring whereas cyclohexene has one ring with one double bond.
Now, we have to consider a test that can differentiate both the compounds easily.
So, let’s take option A first i.e, Bromine water which is an alkene test.
When cyclic cyclohexane is reacted with Bromine water then no reaction occurs but when cyclic cyclohexene reacts with bromine water then it gets reacted with bromine water and an alkene with -Br and -OH bond is formed.
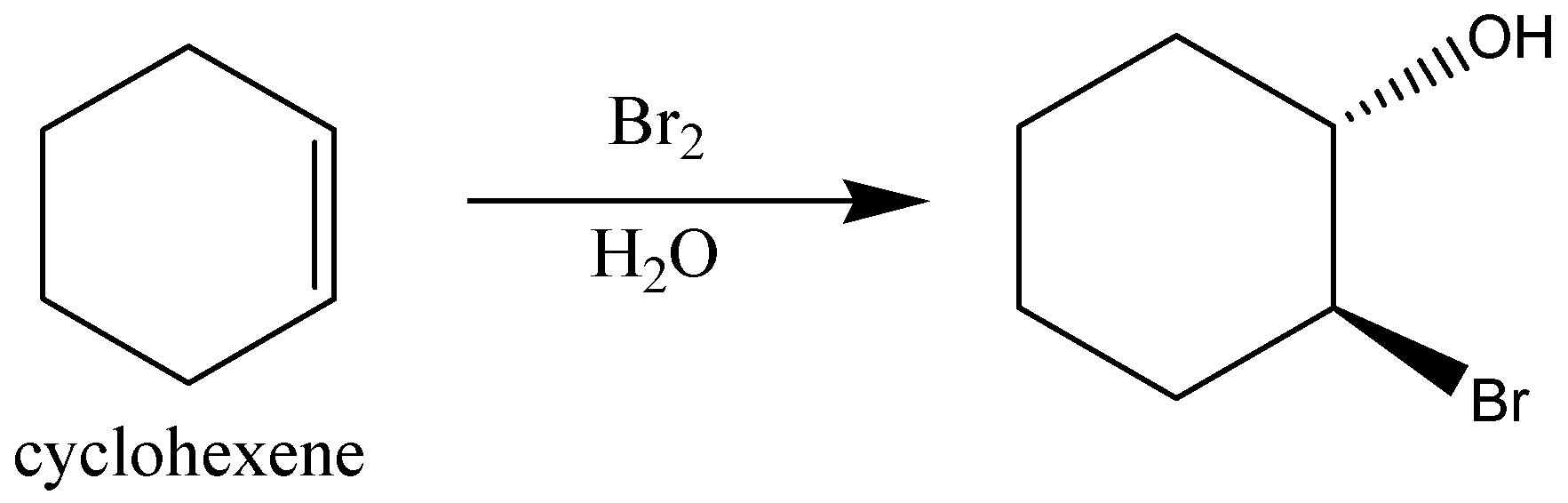
Thus, the main difference with $B{r_2}/{H_2}O$ is that cyclic cyclohexane will not react and cyclic cyclohexene will react with Bromine water.
Now, let’s consider with option B i.e, Bayer’s reagent (alkaline $KMn{O_4}$ solution)
Similarly, with alkaline $KMn{O_4}$ solution, cyclic cyclohexane will not react whereas; cyclic cyclohexene will react and form a compound with –OH and –OH bonds.
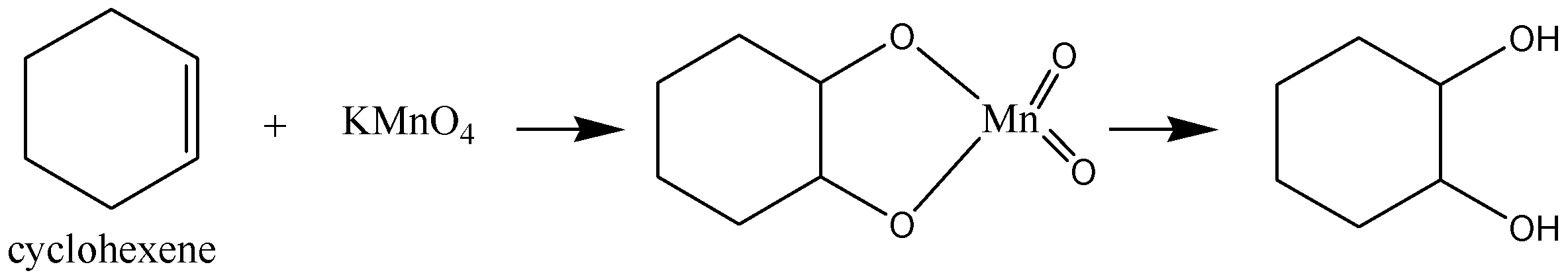
Thus, cyclohexane remains unreacted with both Bromine water and Baeyer's reagent. And cyclohexene reacts with both Bromine water and Baeyer's reagent.
So, the answer will be both option A and B.
Note:
Before performing this test we need to know how to differentiate between cyclic alkane compounds and cyclic alkene compounds using bromine water. Alkenes are unsaturated and decolourises when reacted with bromine water whereas alkanes remain unreacted.
Also, when $KMn{O_4}$ reacts with alkene it converts the purple colour solution to brown in colour and alkane remains unreacted with $KMn{O_4}$ solution.

Complete step by step answer:
Cyclic cyclohexane is ${1^ \circ }$ saturated hydrocarbon and cyclic cyclohexene is ${2^ \circ }$ unsaturated hydrocarbon as cyclohexane has one ring whereas cyclohexene has one ring with one double bond.
Now, we have to consider a test that can differentiate both the compounds easily.
So, let’s take option A first i.e, Bromine water which is an alkene test.
When cyclic cyclohexane is reacted with Bromine water then no reaction occurs but when cyclic cyclohexene reacts with bromine water then it gets reacted with bromine water and an alkene with -Br and -OH bond is formed.

Thus, the main difference with $B{r_2}/{H_2}O$ is that cyclic cyclohexane will not react and cyclic cyclohexene will react with Bromine water.
Now, let’s consider with option B i.e, Bayer’s reagent (alkaline $KMn{O_4}$ solution)
Similarly, with alkaline $KMn{O_4}$ solution, cyclic cyclohexane will not react whereas; cyclic cyclohexene will react and form a compound with –OH and –OH bonds.

Thus, cyclohexane remains unreacted with both Bromine water and Baeyer's reagent. And cyclohexene reacts with both Bromine water and Baeyer's reagent.
So, the answer will be both option A and B.
Note:
Before performing this test we need to know how to differentiate between cyclic alkane compounds and cyclic alkene compounds using bromine water. Alkenes are unsaturated and decolourises when reacted with bromine water whereas alkanes remain unreacted.
Also, when $KMn{O_4}$ reacts with alkene it converts the purple colour solution to brown in colour and alkane remains unreacted with $KMn{O_4}$ solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




