
Give chemical name, formula, and uses of washing soda.
Answer
565.5k+ views
Hint: Washing soft drink includes either as a fixing in some cleaning items or as an independent cleaning specialist. It may very well be made by placing preparing soft drinks in a stove and is regularly utilized in hand crafted cleanser plans, and is in some cases utilized as a clothing supporter or water conditioner. It's an antacid substance that can be utilized for general family unit cleaning, and in any event, for the clothing.
Complete step by step answer:
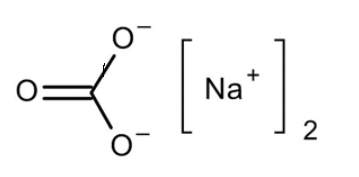
Washing soft drink discovers its application from various perspectives, be it from family unit uses to an immense scope of mechanical applications. It is an antacid compound with a high soluble character which has the capacity to eliminate resolute stains from garments during washing. Washing soft drink equation is composed as \[N{a_2}C{O_3}.10{H_2}O\]. The compound name of washing soft drinks is sodium carbonate. Artificially soft drink debris is a hydrated salt of sodium carbonate.
Solvay Process-Preparation of Sodium Carbonate:
Steps associated with the production of sodium carbonate are clarified beneath:
1.Refinement of Brine
2.Arrangement of sodium hydrogen carbonate
3.Arrangement of sodium carbonate
4.Recuperation of alkali
Stage 1: Purification of Brine
Concentrated brackish water is acquired by the cycle of dissipation and pollution like calcium, magnesium, and so forth are taken out by the precipitation cycle. The concentrated salt water arrangement goes through filtration and is blended in with alkali in the smelling salts tower and the smelling salts tower gets cooled.
Stage 2: Formation of sodium hydrogen carbonate
In a carbonate tower, carbon dioxide is gone through an ammoniated salt water arrangement.
\[N{H_3}(aq)\; + \;C{O_2}(g)\; + \;NaCl(aq)\; + {H_2}O\; \to \;NaHC{O_3}(s)\; + \;N{H_4}Cl(aq)\]
Stage 3: Formation of sodium carbonate
Sodium Bicarbonate \[NaHC{O_{3\;}}\] shaped is obtained from the pinnacle and is warmed at a temperature of 300°C. Thus, developments of sodium carbonate occur.
\[2NaHC{O_{3\;}} \to \;N{a_2}C{O_3}\; + \;C{O_2}\; + \;{H_2}O\]
Stage 4: Recovery of smelling salts
Smelling salts can be recuperated by treating the arrangement of \[N{H_4}Cl\] with \[Ca{(OH)_{2\;}}\]. This alkali is again utilized in the Solvay cycle and \[\;CaC{l_2}\] is gotten as a result.
\[2N{H_4}Cl\; + \;Ca{(OH)_{2\;}} \to \;2N{H_3}\; + \;CaC{l_2}\; + \;{H_2}O\]
Uses of Washing Soda:
1.Utilized as a purging specialist in ventures and family.
2.It discovers its application in paper, material, cleanser, and cleanser ventures.
3.It is utilized during the time spent mellowing of water.
4.It is utilized in the assembling of glass.
5.It is one of the main specialists in laundries.
Note: Washing pop, otherwise known as sodium carbonate (or soft drink debris), is a characteristic cleaner and an incredible water conditioner. It's essential with a pH of 11. The Environmental Working Group gives it "A" on their scale, so it passes without a hitch, making it protected and non-poisonous. In any case, it's extremely harsh and not consumable.
Complete step by step answer:
Washing soft drink discovers its application from various perspectives, be it from family unit uses to an immense scope of mechanical applications. It is an antacid compound with a high soluble character which has the capacity to eliminate resolute stains from garments during washing. Washing soft drink equation is composed as \[N{a_2}C{O_3}.10{H_2}O\]. The compound name of washing soft drinks is sodium carbonate. Artificially soft drink debris is a hydrated salt of sodium carbonate.

Solvay Process-Preparation of Sodium Carbonate:
Steps associated with the production of sodium carbonate are clarified beneath:
1.Refinement of Brine
2.Arrangement of sodium hydrogen carbonate
3.Arrangement of sodium carbonate
4.Recuperation of alkali
Stage 1: Purification of Brine
Concentrated brackish water is acquired by the cycle of dissipation and pollution like calcium, magnesium, and so forth are taken out by the precipitation cycle. The concentrated salt water arrangement goes through filtration and is blended in with alkali in the smelling salts tower and the smelling salts tower gets cooled.
Stage 2: Formation of sodium hydrogen carbonate
In a carbonate tower, carbon dioxide is gone through an ammoniated salt water arrangement.
\[N{H_3}(aq)\; + \;C{O_2}(g)\; + \;NaCl(aq)\; + {H_2}O\; \to \;NaHC{O_3}(s)\; + \;N{H_4}Cl(aq)\]
Stage 3: Formation of sodium carbonate
Sodium Bicarbonate \[NaHC{O_{3\;}}\] shaped is obtained from the pinnacle and is warmed at a temperature of 300°C. Thus, developments of sodium carbonate occur.
\[2NaHC{O_{3\;}} \to \;N{a_2}C{O_3}\; + \;C{O_2}\; + \;{H_2}O\]
Stage 4: Recovery of smelling salts
Smelling salts can be recuperated by treating the arrangement of \[N{H_4}Cl\] with \[Ca{(OH)_{2\;}}\]. This alkali is again utilized in the Solvay cycle and \[\;CaC{l_2}\] is gotten as a result.
\[2N{H_4}Cl\; + \;Ca{(OH)_{2\;}} \to \;2N{H_3}\; + \;CaC{l_2}\; + \;{H_2}O\]
Uses of Washing Soda:
1.Utilized as a purging specialist in ventures and family.
2.It discovers its application in paper, material, cleanser, and cleanser ventures.
3.It is utilized during the time spent mellowing of water.
4.It is utilized in the assembling of glass.
5.It is one of the main specialists in laundries.
Note: Washing pop, otherwise known as sodium carbonate (or soft drink debris), is a characteristic cleaner and an incredible water conditioner. It's essential with a pH of 11. The Environmental Working Group gives it "A" on their scale, so it passes without a hitch, making it protected and non-poisonous. In any case, it's extremely harsh and not consumable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




