
Give IUPAC name of the expected product in the given reaction:
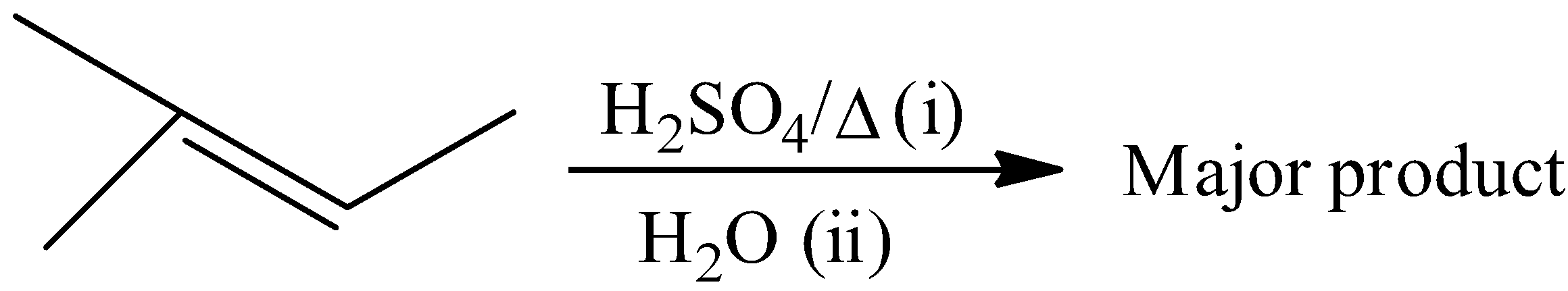
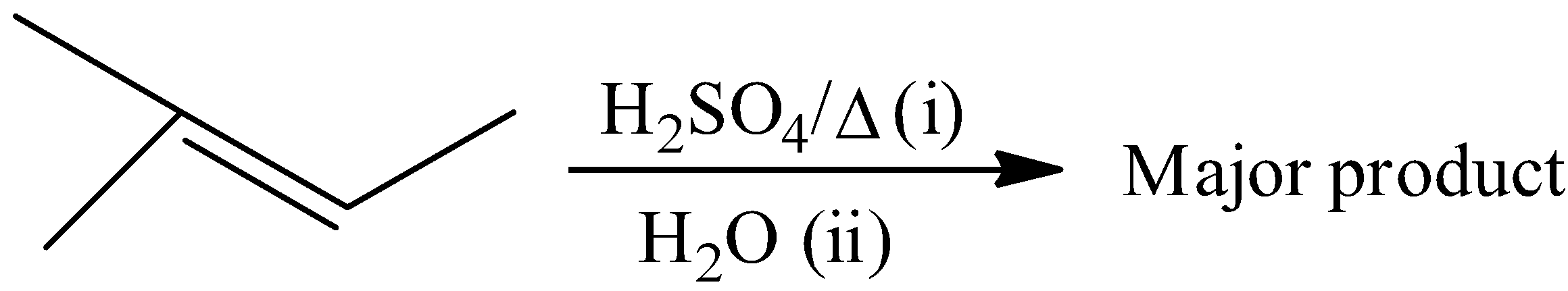
Answer
565.5k+ views
Hint:We have to know that alkenes undergo electrophilic addition reactions with concentration sulfuric acid. Alkyl hydrogen sulfates are the products formed when alkenes react with concentrated sulfuric acid. This also involves the conversion of the product into an alcohol.
Complete step by step answer:
The reactant of the given reaction is identified as an alkene. The given reactant is 2-methyl-2-butene. The mechanism for the reaction between 2-methyl-2-butene and sulfuric acid is that the atoms of hydrogen are linked to very electronegative oxygen atoms that signify that the hydrogens would contain slight positive charge while the oxygen would be slightly negative. In the mechanism, we will notice one of the hydrogen to oxygen bonds, because the other one is too far from the carbon-carbon double bond to be involved in any way. When 2-methyl-2-butene reacts with sulfuric acid the product would be 2-methyl-2-butyl hydrogen sulfate. We can write the reaction is,
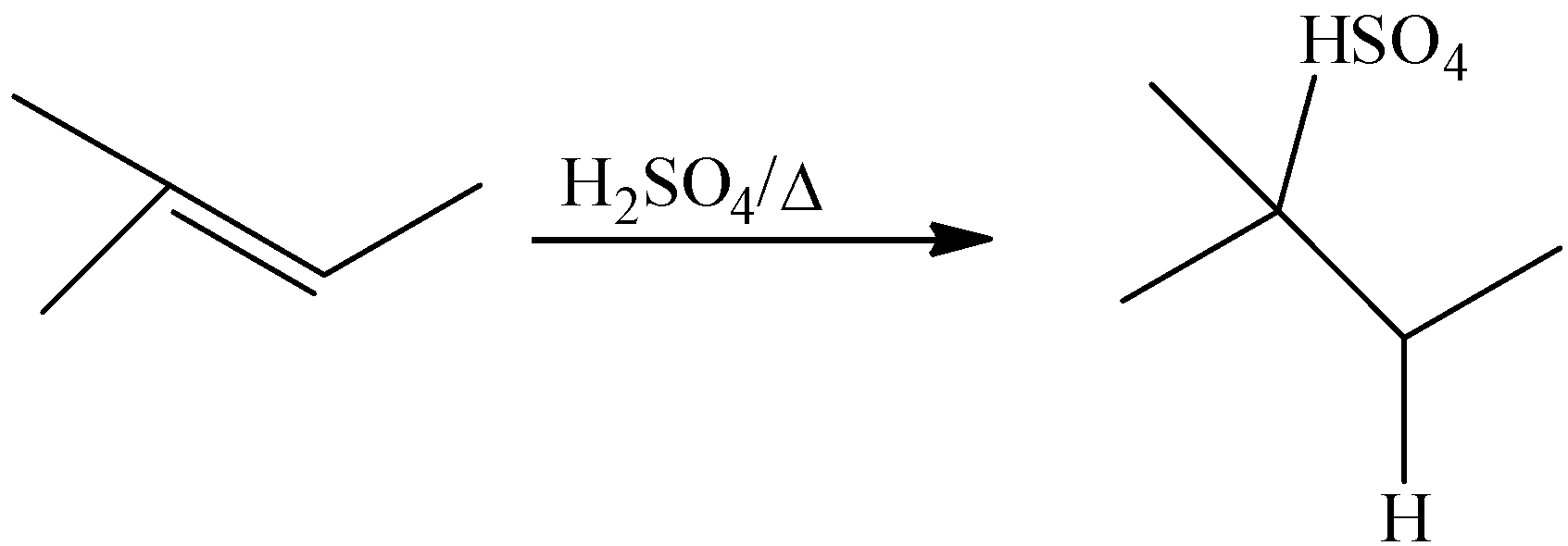
The formed 2-methyl-2-butyl hydrogen sulfate is then hydrolyzed in the presence of water, and then the product formed would be alcohol. We could write the reaction as,
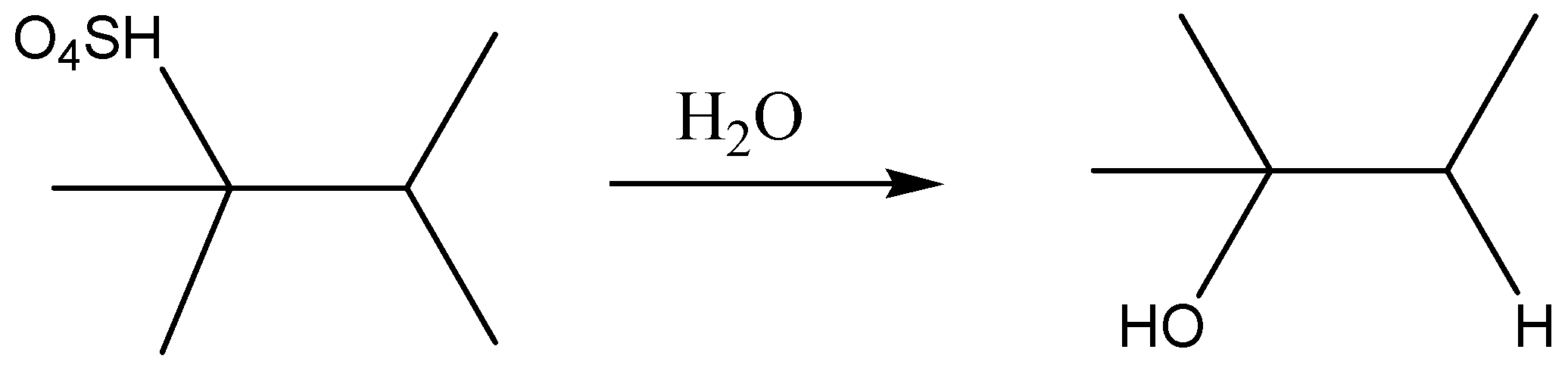
The product formed would be 2-methyl-2-butyl alcohol.
We could write the IUPAC name of alcohol by following rules:
-The longest continuous chain of carbon atoms having the hydroxyl group is taken as the parent compound. We have to number the chain from the terminal adjacent to the hydroxyl group.
-The number that represents the location of the hydroxyl group is prefixed to the name of the parent hydrocarbon, and the -e ending of the parent alkane is displaced by the suffix -ol. We have to name and number the substituents similar in alkanes.
-If more than one hydroxyl group looks in the same molecule suffixes like -diol and -triol are employed. In these cases, the -e ending of the parent alkane is kept.
The product is,
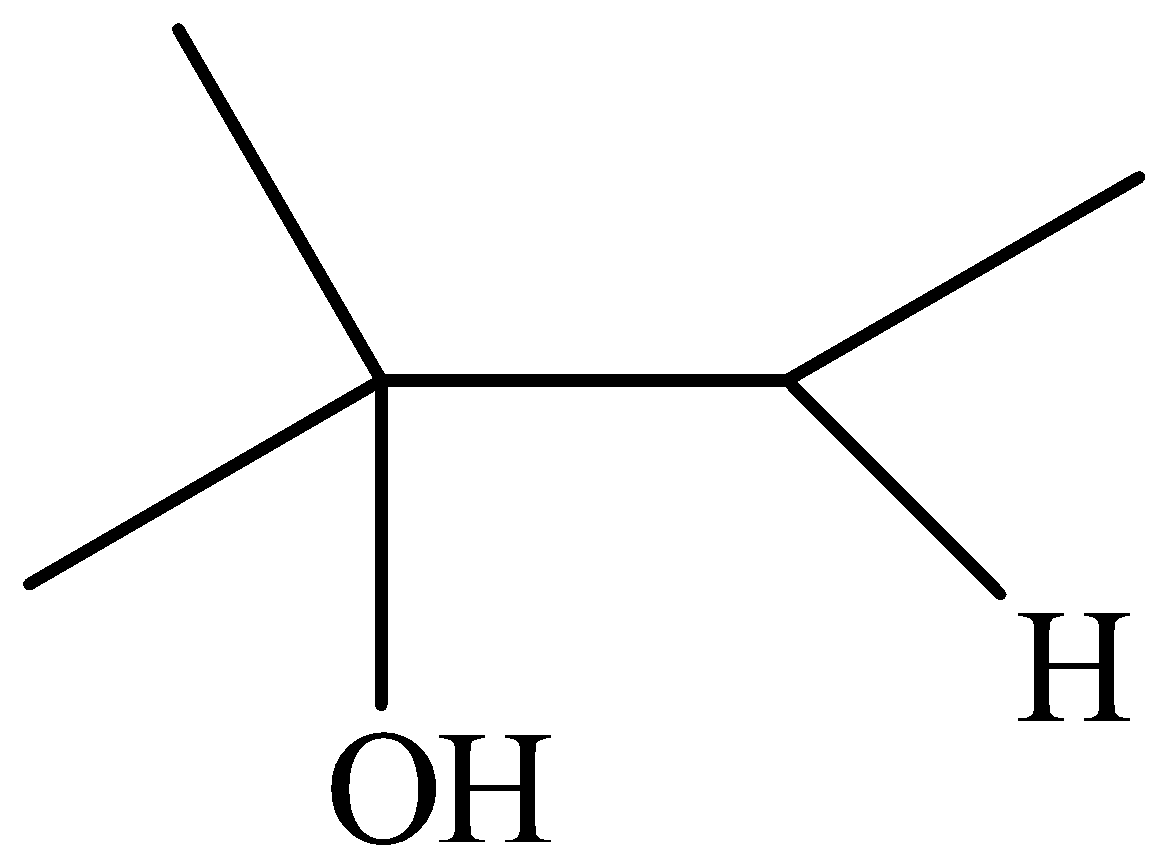
We can write the structural formula of the product as,
From the structural formula, the parent compound is butane, the -e ending of the parent alkane is displaced by the suffix –ol. We can see that the hydroxyl group is seen at second position and the methyl group is seen at second position.
So, IUPAC name of the compound is 2-methyl-2-butanol.
Note:
We know that organic compounds are much needed because they contain carbon in all living organisms. They are the basic units which move the world in several of the cycles. For example: The carbon cycle involves exchanging carbon in photosynthesis and respiration of cells between plants and animals. Ethanol and isopropanol are employed as antiseptics and carboxylic acids are employed in pharmaceuticals.
Complete step by step answer:
The reactant of the given reaction is identified as an alkene. The given reactant is 2-methyl-2-butene. The mechanism for the reaction between 2-methyl-2-butene and sulfuric acid is that the atoms of hydrogen are linked to very electronegative oxygen atoms that signify that the hydrogens would contain slight positive charge while the oxygen would be slightly negative. In the mechanism, we will notice one of the hydrogen to oxygen bonds, because the other one is too far from the carbon-carbon double bond to be involved in any way. When 2-methyl-2-butene reacts with sulfuric acid the product would be 2-methyl-2-butyl hydrogen sulfate. We can write the reaction is,

The formed 2-methyl-2-butyl hydrogen sulfate is then hydrolyzed in the presence of water, and then the product formed would be alcohol. We could write the reaction as,

The product formed would be 2-methyl-2-butyl alcohol.
We could write the IUPAC name of alcohol by following rules:
-The longest continuous chain of carbon atoms having the hydroxyl group is taken as the parent compound. We have to number the chain from the terminal adjacent to the hydroxyl group.
-The number that represents the location of the hydroxyl group is prefixed to the name of the parent hydrocarbon, and the -e ending of the parent alkane is displaced by the suffix -ol. We have to name and number the substituents similar in alkanes.
-If more than one hydroxyl group looks in the same molecule suffixes like -diol and -triol are employed. In these cases, the -e ending of the parent alkane is kept.
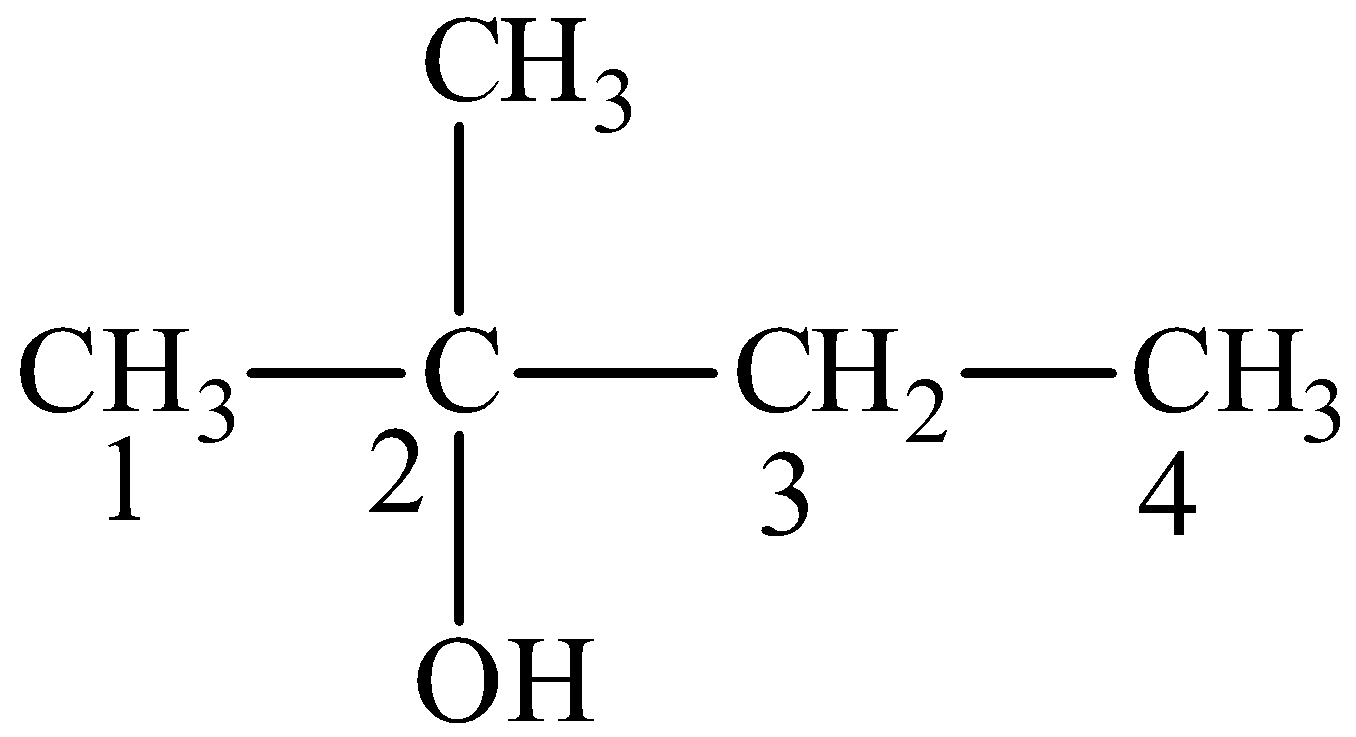
The product is,

We can write the structural formula of the product as,

From the structural formula, the parent compound is butane, the -e ending of the parent alkane is displaced by the suffix –ol. We can see that the hydroxyl group is seen at second position and the methyl group is seen at second position.
So, IUPAC name of the compound is 2-methyl-2-butanol.
Note:
We know that organic compounds are much needed because they contain carbon in all living organisms. They are the basic units which move the world in several of the cycles. For example: The carbon cycle involves exchanging carbon in photosynthesis and respiration of cells between plants and animals. Ethanol and isopropanol are employed as antiseptics and carboxylic acids are employed in pharmaceuticals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




