
Give one example of a tetra atomic molecule.
Answer
531.5k+ views
Hint: Tetra atomic molecules are those molecules that are formed when four atoms chemically bond together. Atomicity of tetra atomic compounds is three where atomicity is the total number of atoms present in one molecule of an element, compound, or a substance.
Complete Step by step solution:
- Tetra atomic molecules are those molecules that are formed by a combination of four atoms.
- There are plenty of tetra atomic molecules available. Ammonia and sulfur trioxide are some examples of tetra-atomic molecules. The molecular formula for ammonia is $N{{H}_{3}}$and for sulfur trioxide is $S{{O}_{3}}$.
- Let’s see the tetra atomic structure of ammonia:
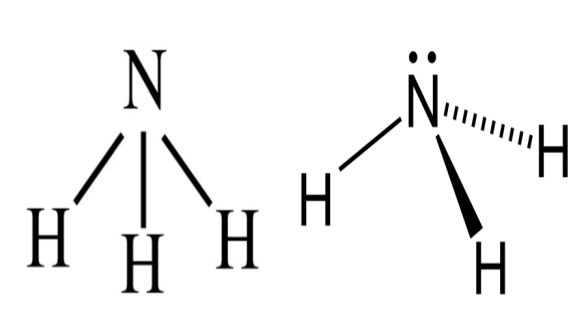 Ammonia
Ammonia
Additional Information:
Let's see the meaning of monoatomic, diatomic, triatomic, tetra atomic, and polyatomic molecules with examples.
- Monoatomic molecules: Molecules that are stable as a single atom are called monatomic molecules. An example is helium gas.
- Diatomic molecules:
Molecules that consist of two atoms are called diatomic molecules. Examples of diatomic molecules are hydrogen, oxygen, etc.
- Triatomic molecules:
Molecules that consist of three atoms are called a triatomic molecule. An example is ozone.
- Tetra atomic molecules:
Molecules that contain 4 atoms are called tetra atomic molecules. An example is ammonia.
- Polyatomic molecules:
Molecules that have four or more than four atoms are called polyatomic molecules. An example is benzene.
Note: Don’t confuse between the number of atoms bound chemically together in tetra atomic molecules and atomicity of tetra atomic molecules. There are four atoms chemically bound together in the tetra atomic molecule with atomicity three.
Complete Step by step solution:
- Tetra atomic molecules are those molecules that are formed by a combination of four atoms.
- There are plenty of tetra atomic molecules available. Ammonia and sulfur trioxide are some examples of tetra-atomic molecules. The molecular formula for ammonia is $N{{H}_{3}}$and for sulfur trioxide is $S{{O}_{3}}$.
- Let’s see the tetra atomic structure of ammonia:

Additional Information:
Let's see the meaning of monoatomic, diatomic, triatomic, tetra atomic, and polyatomic molecules with examples.
- Monoatomic molecules: Molecules that are stable as a single atom are called monatomic molecules. An example is helium gas.
- Diatomic molecules:
Molecules that consist of two atoms are called diatomic molecules. Examples of diatomic molecules are hydrogen, oxygen, etc.
- Triatomic molecules:
Molecules that consist of three atoms are called a triatomic molecule. An example is ozone.
- Tetra atomic molecules:
Molecules that contain 4 atoms are called tetra atomic molecules. An example is ammonia.
- Polyatomic molecules:
Molecules that have four or more than four atoms are called polyatomic molecules. An example is benzene.
Note: Don’t confuse between the number of atoms bound chemically together in tetra atomic molecules and atomicity of tetra atomic molecules. There are four atoms chemically bound together in the tetra atomic molecule with atomicity three.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




