
Give reason why half-filled or fully filled orbitals provide more stability to the molecules?
Answer
585k+ views
Hint: Every orbital of an atom has a capacity to take two electrons. The two electrons in each orbital of the same atom have similar energy but have different spins. Means no two electrons have the same configuration in an atom according to Pauli’s exclusion principle.
Complete step by step answer:
- Very few elements in the periodic table have half-filled or fully filled orbitals.
- Due to half-filled or fully filled orbitals the atom gets greater stability than other electronic configurations.
- The reasons behind the greater stability of the atoms having half-filled or fully filled orbitals are symmetry and exchange energy.
- We know that half-filled or fully filled atomic orbitals have more symmetry than any other electronic configuration and this symmetry leads to the greater stability of the atom.
- Half-filled and fully filled atomic orbitals can be represented as follows.
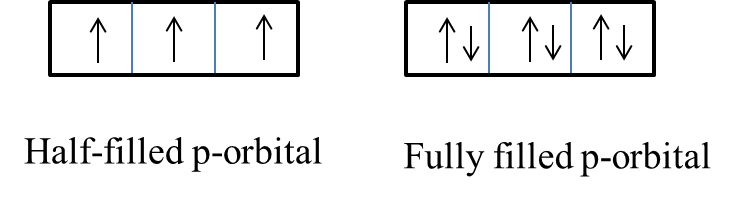
- From the above picture we can see the p-orbital with half-filled or fully filled electrons has maximum symmetry.
- The electrons located in different orbitals of the matching sub- shell can interchange their positions. Each such interchange of electrons decreases the energy of the atom known as exchange energy.
- If the exchange of the electrons is greater automatically the exchange energy will be greater and stability will be greater.
- The number of exchanges between electrons in half-filled and fully filled orbitals are maximum then stability will be maximum.
- The reason behind stability of the half-filled and fully filled electrons in orbitals is symmetry and exchange energy of the electrons.
Note: Chromium is the element that has half-filled electrons in 3d subshell and shows great stability. Copper is the element that has fully filled electrons in 3d subshell and shows great stability.
Electronic configuration of chromium is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}4{{s}^{1}}$
Electronic configuration of copper is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{1}}$
Complete step by step answer:
- Very few elements in the periodic table have half-filled or fully filled orbitals.
- Due to half-filled or fully filled orbitals the atom gets greater stability than other electronic configurations.
- The reasons behind the greater stability of the atoms having half-filled or fully filled orbitals are symmetry and exchange energy.
- We know that half-filled or fully filled atomic orbitals have more symmetry than any other electronic configuration and this symmetry leads to the greater stability of the atom.
- Half-filled and fully filled atomic orbitals can be represented as follows.

- From the above picture we can see the p-orbital with half-filled or fully filled electrons has maximum symmetry.
- The electrons located in different orbitals of the matching sub- shell can interchange their positions. Each such interchange of electrons decreases the energy of the atom known as exchange energy.
- If the exchange of the electrons is greater automatically the exchange energy will be greater and stability will be greater.
- The number of exchanges between electrons in half-filled and fully filled orbitals are maximum then stability will be maximum.
- The reason behind stability of the half-filled and fully filled electrons in orbitals is symmetry and exchange energy of the electrons.
Note: Chromium is the element that has half-filled electrons in 3d subshell and shows great stability. Copper is the element that has fully filled electrons in 3d subshell and shows great stability.
Electronic configuration of chromium is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}4{{s}^{1}}$
Electronic configuration of copper is: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{1}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




