
Give reasons.
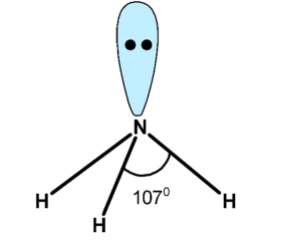
(i) Bond angle of $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$ is $\text{ 10}{{\text{7}}^{\text{0}}}\text{ }$ .
(ii) In $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$ , $\text{B}-\text{F}$ the bond is polar but $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$has a zero dipole moment.
Answer
577.8k+ views
Hint: (i) The bond angle of a molecule depends on the hybridization of the central atom. According to VSEPR theory, electron pairs that are bond pairs, lone pairs repel each other. This may lead to the increase or decrease in the bond angle from the expected values. The nitrogen atom has 5 electrons in its outermost shell \[\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{3 }}}\]. These electrons take part in the formation of electron pairs.
(ii) Dipole moment is a measure of the polarity of a bond.it is a vector quantity .the direction of the dipole is always towards the electronegative atom in the bond in $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$, Fluorine is an electronegative compare to boron and attracts the electron density towards it. This polarises the bond and gives it a non-zero dipole moment.
Complete step by step solution:
(i) Lets first determine the hybridization and structure of the ammonia $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$ molecule. Central atom nitrogen has the electronic configuration as,
$\text{ N = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{3 }}}$
One 2s and three 2p orbital combines and to form 4 $\text{ s}{{\text{p}}^{\text{3}}}\text{ }$ hybridized orbitals. Out of these 4 orbitals,3 hybridized orbitals form three bonds with hydrogen atoms. The fourth hybridized orbital contains two electrons in it. It holds a lone pair. If we compare it with methane $\text{ C}{{\text{H}}_{\text{4}}}\text{ }$ expected bond angle is $\text{ 109}\text{.}{{\text{5}}^{\text{0}}}\text{ }$. However, due to non-bonding or lone pairs, has a high electron density and pushes the three bonding pairs and thus reduces the bond angle from $\text{ 109}\text{.}{{\text{5}}^{\text{0}}}\text{ }$to$\text{ 10}{{\text{7}}^{\text{0}}}\text{ }$.
(ii) The boron trifluoride $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$ has a trigonal planar geometry. Here, each $\text{B}-\text{F}$ is orientated towards the three corners of the triangle. Three fluorine atoms are attached to the boron. The fluorine is electronegative thus it attracts the bonded electrons towards it. If in a molecule, dipole moments are in the opposite direction and have the same direction, they cancel out each other.
Thus the dipole moment of each $\text{B}-\text{F}$ bond is towards the fluorine. As shown below,

The three fluorine atoms are attached to the boron atom. These bonds are directed towards the corners of the triangle. Thus the resultant dipole moment of the two fluorine atoms is cancelled out by the dipole moment of the third $\text{B}-\text{F}$ bond.
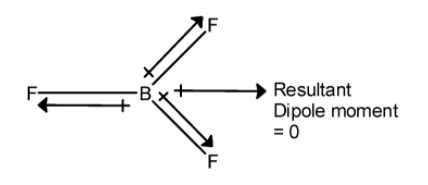
Thus the resultant dipole moment of $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$ the molecule is zero.
Note: (i) The lone pair lone pair repulsion is maximum followed by the bond pair lone pair repulsion. The bond pair-bond pair experiences least repulsion .the increasing order of electron pair repulsion is as follows,
$\text{ LP}-\text{LP }>\text{ LP}-\text{BP }>\text{ BP}-\text{BP }$
(ii)Note that the dipole moment is directly related to the difference in the electronegativity of the atoms forming a bonding. Larger the difference the more polarised the bond and the higher is the dipole moment.
(ii) Dipole moment is a measure of the polarity of a bond.it is a vector quantity .the direction of the dipole is always towards the electronegative atom in the bond in $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$, Fluorine is an electronegative compare to boron and attracts the electron density towards it. This polarises the bond and gives it a non-zero dipole moment.
Complete step by step solution:
(i) Lets first determine the hybridization and structure of the ammonia $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$ molecule. Central atom nitrogen has the electronic configuration as,
$\text{ N = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{3 }}}$
One 2s and three 2p orbital combines and to form 4 $\text{ s}{{\text{p}}^{\text{3}}}\text{ }$ hybridized orbitals. Out of these 4 orbitals,3 hybridized orbitals form three bonds with hydrogen atoms. The fourth hybridized orbital contains two electrons in it. It holds a lone pair. If we compare it with methane $\text{ C}{{\text{H}}_{\text{4}}}\text{ }$ expected bond angle is $\text{ 109}\text{.}{{\text{5}}^{\text{0}}}\text{ }$. However, due to non-bonding or lone pairs, has a high electron density and pushes the three bonding pairs and thus reduces the bond angle from $\text{ 109}\text{.}{{\text{5}}^{\text{0}}}\text{ }$to$\text{ 10}{{\text{7}}^{\text{0}}}\text{ }$.

(ii) The boron trifluoride $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$ has a trigonal planar geometry. Here, each $\text{B}-\text{F}$ is orientated towards the three corners of the triangle. Three fluorine atoms are attached to the boron. The fluorine is electronegative thus it attracts the bonded electrons towards it. If in a molecule, dipole moments are in the opposite direction and have the same direction, they cancel out each other.
Thus the dipole moment of each $\text{B}-\text{F}$ bond is towards the fluorine. As shown below,

The three fluorine atoms are attached to the boron atom. These bonds are directed towards the corners of the triangle. Thus the resultant dipole moment of the two fluorine atoms is cancelled out by the dipole moment of the third $\text{B}-\text{F}$ bond.

Thus the resultant dipole moment of $\text{ B}{{\text{F}}_{\text{3}}}\text{ }$ the molecule is zero.
Note: (i) The lone pair lone pair repulsion is maximum followed by the bond pair lone pair repulsion. The bond pair-bond pair experiences least repulsion .the increasing order of electron pair repulsion is as follows,
$\text{ LP}-\text{LP }>\text{ LP}-\text{BP }>\text{ BP}-\text{BP }$
(ii)Note that the dipole moment is directly related to the difference in the electronegativity of the atoms forming a bonding. Larger the difference the more polarised the bond and the higher is the dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




