
Give some examples of Tyndall effect observed in your surroundings? This question has multiple correct options
A. Sunbeam coming from ventilation
B. Milk in a glass appearing faint blue
C. Sunbeam coming from behind the clouds
D. Flour suspended in water
Answer
599.1k+ views
Hint: To answer this question, we should be updated on the basis of the knowledge about light scattering and dispersion. We see Tyndall effect in our surroundings on a daily basis though it is kept unnoticed by us.
Step by step answer:
Tyndall effect can be defined as the phenomenon where scattering of light by colloidal particles takes place and the path of light is made visible through dispersion. Some examples of Tyndall effect are:
1. Sunlight entering into a dark room.
2. Lots of dust particles suspended in a lit up room.
3. When the weather is foggy and smoggy, beams of headlights are clearly visible.
4. Scattering of light by water droplets present in the air.
In the options mentioned in the question, the first option, that is, sunbeam coming from ventilation is a classic example of Tyndall Effect because of the visibility of light beams.
The second option, milk in a glass appearing faint blue cannot be considered as an example of Tyndall effect as light is scattered by the colloidal particles present inside milk giving it a blue shade when light passes through it. Therefore, rendering the light beams invisible.
The third option, sunbeam coming from behind the clouds is also an example of Tyndall effect as the particles present inside the clouds causes dispersion of light making beams of light visible.
The fourth option, flour suspended in water also causes dispersion of light after refraction in water and therefore light tends to bend in the medium with its beams visible. Hence, this is also an example of Tyndall effect.
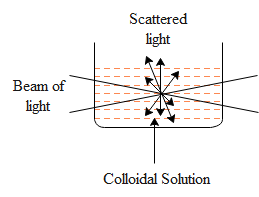
Therefore, the correct options are Option A, Option C and Option D.
Note: We should know that in lyophobic colloids, the difference is appreciable and therefore, the Tyndall effect is well defined. This effect is used to determine whether a mixture is a true solution or a colloid.
Step by step answer:
Tyndall effect can be defined as the phenomenon where scattering of light by colloidal particles takes place and the path of light is made visible through dispersion. Some examples of Tyndall effect are:
1. Sunlight entering into a dark room.
2. Lots of dust particles suspended in a lit up room.
3. When the weather is foggy and smoggy, beams of headlights are clearly visible.
4. Scattering of light by water droplets present in the air.
In the options mentioned in the question, the first option, that is, sunbeam coming from ventilation is a classic example of Tyndall Effect because of the visibility of light beams.
The second option, milk in a glass appearing faint blue cannot be considered as an example of Tyndall effect as light is scattered by the colloidal particles present inside milk giving it a blue shade when light passes through it. Therefore, rendering the light beams invisible.
The third option, sunbeam coming from behind the clouds is also an example of Tyndall effect as the particles present inside the clouds causes dispersion of light making beams of light visible.
The fourth option, flour suspended in water also causes dispersion of light after refraction in water and therefore light tends to bend in the medium with its beams visible. Hence, this is also an example of Tyndall effect.

Therefore, the correct options are Option A, Option C and Option D.
Note: We should know that in lyophobic colloids, the difference is appreciable and therefore, the Tyndall effect is well defined. This effect is used to determine whether a mixture is a true solution or a colloid.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




