
Give the formula of magnesium carbonate.
Answer
578.4k+ views
Hint:We know that magnesium carbonate is an inorganic salt that is $MgC{O_3}$ and it is also known as Magnesite or Hydromagnesite or Barringtonite. Hydrated magnesite is present as minerals and they serve as a fertilizer and as an antacid. It’s extensively used in the preparation of materials that are capable of withstanding very elevated temperatures.
Complete step by step answer:
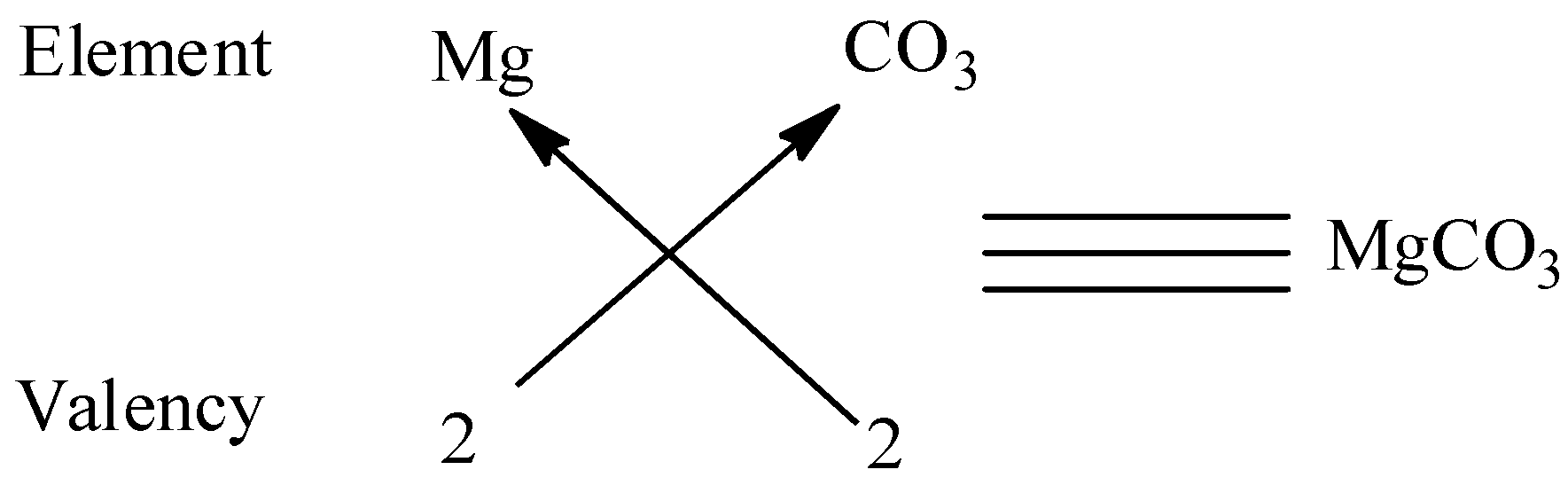
We know that the valency of carbonate \[2\] and the valency of magnesium are \[2\] and to satisfy the combining capacity of carbonate, one magnesium is required. Thus the molecular formula is $MgC{O_3}$.
Additional notes:
We also know that the magnesium carbonate may be a basic hydrated magnesium carbonate or a usually hydrated magnesium carbonate. It presents as a light, white, friable mass or as a bulky white powder. It is neutral and is constant in air. It’s almost insoluble in water to which though it imparts a somewhat alkaline reaction. It is insoluble in alcohol but is dissolved by dilute acids with bubbles. Hydromagnesite may be a white or yellowish or grayish-white or brown colored compound which is produced from crystalline powder or crystalline solid form. It’s a vital ore for magnesium.
Note: Now we discuss about the some of the uses of Magnesium carbonate as:
- Magnesium carbonate is employed in food as a desiccant.
- Utilized in making pharmaceutical products.
- Utilized as a rubber reinforcing agent.
- Utilized in the manufacturing of cosmetics.
- Utilized in making drinking water.
- Utilized as an anti caking agent in food.
- Utilized as a filtering agent.
- Utilized as fire-extinguishing.
- Utilized in printing inks.
Complete step by step answer:
We know that the valency of carbonate \[2\] and the valency of magnesium are \[2\] and to satisfy the combining capacity of carbonate, one magnesium is required. Thus the molecular formula is $MgC{O_3}$.

Additional notes:
We also know that the magnesium carbonate may be a basic hydrated magnesium carbonate or a usually hydrated magnesium carbonate. It presents as a light, white, friable mass or as a bulky white powder. It is neutral and is constant in air. It’s almost insoluble in water to which though it imparts a somewhat alkaline reaction. It is insoluble in alcohol but is dissolved by dilute acids with bubbles. Hydromagnesite may be a white or yellowish or grayish-white or brown colored compound which is produced from crystalline powder or crystalline solid form. It’s a vital ore for magnesium.
Note: Now we discuss about the some of the uses of Magnesium carbonate as:
- Magnesium carbonate is employed in food as a desiccant.
- Utilized in making pharmaceutical products.
- Utilized as a rubber reinforcing agent.
- Utilized in the manufacturing of cosmetics.
- Utilized in making drinking water.
- Utilized as an anti caking agent in food.
- Utilized as a filtering agent.
- Utilized as fire-extinguishing.
- Utilized in printing inks.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




