
Give the IUPAC name of ${\left( {{{CC}}{{{l}}_3}} \right)_3}{{CCl}}$.
Answer
565.5k+ views
Hint: This compound is named as perchloric isobutane. Another name of this compound is tris-(trichloromethyl) chloromethane. It has a molecular formula of ${{{C}}_4}{{C}}{{{l}}_{10}}$. The molecular weight of this compound is ${{402}}{{.54g}}$.
Complete step by step answer:
The structure of this compound is given below:
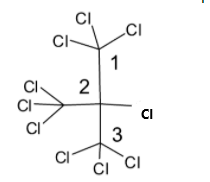
There are only halogens as functional groups. So we have to find the longest carbon chain or the parent chain. The carbon with three chlorine atoms is the first carbon. The middle carbon atom which has a chlorine atom and three trichlormethyl group is the second carbon atom. Third carbon is another carbon atom which has three chlorine atoms attached. The word root contains three carbon atoms. So the prefix is used as “prop” and “ane” is used because there are no double or triple bonds, but only single bonds. We can see in the structure given above that there are a total of seven chlorine atoms. Three are attached to the first carbon atom, one is attached to the second carbon atom and three are attached to the third carbon atom. So the IUPAC name will be $2 - $ trichloromethyl $ - 1,1,1,2,3,3,3 - $ heptafluoropropane. Since a trichloromethyl group is attached to second carbon, it is named as $2 - $ trichloromethyl. The substitutions of chlorine atoms in the carbon atom is represented as $ - 1,1,1,2,3,3,3 - $.
Note: The given compound is actually a isobutane molecule in which the hydrogen atoms are substituted with chlorine atoms. Thus it has a common name perchloric isobutane. We have named the compound by counting the number of carbon atoms in the parent chain followed by the primary suffix and prefix.
Complete step by step answer:
The structure of this compound is given below:

There are only halogens as functional groups. So we have to find the longest carbon chain or the parent chain. The carbon with three chlorine atoms is the first carbon. The middle carbon atom which has a chlorine atom and three trichlormethyl group is the second carbon atom. Third carbon is another carbon atom which has three chlorine atoms attached. The word root contains three carbon atoms. So the prefix is used as “prop” and “ane” is used because there are no double or triple bonds, but only single bonds. We can see in the structure given above that there are a total of seven chlorine atoms. Three are attached to the first carbon atom, one is attached to the second carbon atom and three are attached to the third carbon atom. So the IUPAC name will be $2 - $ trichloromethyl $ - 1,1,1,2,3,3,3 - $ heptafluoropropane. Since a trichloromethyl group is attached to second carbon, it is named as $2 - $ trichloromethyl. The substitutions of chlorine atoms in the carbon atom is represented as $ - 1,1,1,2,3,3,3 - $.
Note: The given compound is actually a isobutane molecule in which the hydrogen atoms are substituted with chlorine atoms. Thus it has a common name perchloric isobutane. We have named the compound by counting the number of carbon atoms in the parent chain followed by the primary suffix and prefix.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




