
Give the IUPAC name of the following compound- Isopropyl alcohol.
Answer
539.4k+ views
Hint: Iso group can be defined as the structural isomer of a straight chain alkane where the second carbon will be attached to a methyl group. When any functional group is attached to the second carbon, the hydrocarbon will be called as a secondary carbon.
Complete step by step solution
Alcohols are the functional group where the hydrocarbon is attached to the $ - OH $ group. The hydrocarbons can include alkanes, alkenes, aromatic compounds, etc.
A Primary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to only one carbon. For example, $ Propan - 1 - ol $ that can be represented as
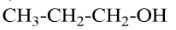
A secondary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to two carbons in the structure. For example, $ Bu\tan - 2 - ol $ that can be represented as
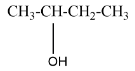
A tertiary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to three carbons in the structure. For example, $ 2 - Methylpropan - 2 - ol $ that can be represented as
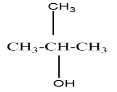
On oxidation of primary alcohol we get an aldehyde. But on oxidising a secondary alcohol, a ketone is usually formed.
The structure of isopropyl alcohol can be represented as-

The IUPAC naming can be done as-
In the first step, as we can see from the above structure that the compound has three carbon in the longest chain and it does not contain double or triple bond, hence the name of the compound will start from $ Propan - $
In the second step, the suffix used to represent an alcoholic group in a compound will be “ $ - ol $ ”.
In the last step the position of alcohol will be noted and that is $ 2 $ from the either side.
Hence the IUPAC name of Isopropyl alcohol will be $ Propan - 2 - ol $ .
Note: When we oxidise the alcohols we get an aldehyde or a ketone depending on the type of carbon where the alcoholic group is attached. On oxidation of primary alcohol we get an aldehyde. But on oxidising a secondary alcohol, a ketone is usually formed like on oxidising isopropyl alcohol with PCC we will get acetone.
Complete step by step solution
Alcohols are the functional group where the hydrocarbon is attached to the $ - OH $ group. The hydrocarbons can include alkanes, alkenes, aromatic compounds, etc.
A Primary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to only one carbon. For example, $ Propan - 1 - ol $ that can be represented as
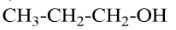
A secondary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to two carbons in the structure. For example, $ Bu\tan - 2 - ol $ that can be represented as

A tertiary alcohol will be said to the compound where the alcoholic group is attached to the carbon which is attached to three carbons in the structure. For example, $ 2 - Methylpropan - 2 - ol $ that can be represented as

On oxidation of primary alcohol we get an aldehyde. But on oxidising a secondary alcohol, a ketone is usually formed.
The structure of isopropyl alcohol can be represented as-

The IUPAC naming can be done as-
In the first step, as we can see from the above structure that the compound has three carbon in the longest chain and it does not contain double or triple bond, hence the name of the compound will start from $ Propan - $
In the second step, the suffix used to represent an alcoholic group in a compound will be “ $ - ol $ ”.
In the last step the position of alcohol will be noted and that is $ 2 $ from the either side.
Hence the IUPAC name of Isopropyl alcohol will be $ Propan - 2 - ol $ .
Note: When we oxidise the alcohols we get an aldehyde or a ketone depending on the type of carbon where the alcoholic group is attached. On oxidation of primary alcohol we get an aldehyde. But on oxidising a secondary alcohol, a ketone is usually formed like on oxidising isopropyl alcohol with PCC we will get acetone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




