
Give the product of oxidation with $Mn{{O}_{2}}$of ${{C}_{6}}{{H}_{5}}CH(OH)C{{H}_{2}}C{{H}_{2}}OH$.
Answer
531.3k+ views
Hint: As we know that allylic and benzylic alcohol can be readily oxidized under mild conditions. Also manganese (IV) dioxide is a mild oxidizing agent which selectively oxidizes primary or secondary allylic alcohols. We will use this information of reagent in the given question.
Complete answer:
Let us first understand the oxidation of allylic and benzylic alcohol with the help of manganese (IV) dioxide:-
-The allylic and benzylic alcohols are oxidized specifically and selectively by a suspension of activated manganese (IV) dioxide ($Mn{{O}_{2}}$). Aldehydes are formed by the oxidation of primary allylic alcohols and ketones are formed by the oxidation of secondary allylic alcohols.
-For this reaction, we require an “activated$Mn{{O}_{2}}$” which is obtained by the oxidation – reduction reaction of potassium permanganate and it is also a mild oxidizing agent. The oxidation of alcohols takes place on the surface of manganese (IV) dioxide ($Mn{{O}_{2}}$) which is insoluble in the solvents used for this reaction.
-The reaction is highly chemoselective because allylic and benzylic alcohols react more rapidly than the ordinary alcohols.
The oxidation reaction of ${{C}_{6}}{{H}_{5}}CH(OH)C{{H}_{2}}C{{H}_{2}}OH$with the help of $Mn{{O}_{2}}$ is shown below:-
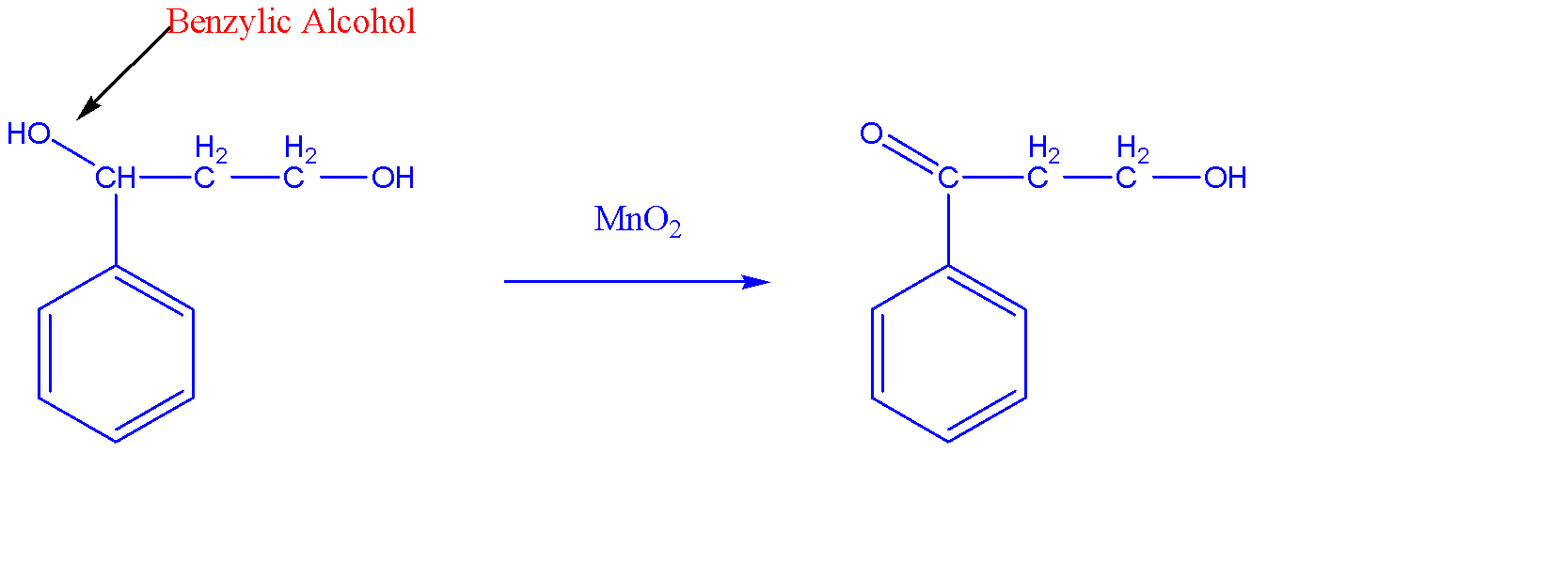
We can see that there are two alcohols in the reactant among which $Mn{{O}_{2}}$have selective nature towards benzylic alcohol and hence it will get oxidized to become a carbonyl compound. Since it is a secondary benzylic alcohol, we obtain ketone as a product.
Note:
The other reagents that can be specially used to oxidize allylic and benzylic alcohol are $Mn{{(OAc)}_{2}}$and $Mn{{(OAc)}_{3}}$.
-Remember that water competes with the alcohol for the sites on the $Mn{{O}_{2}}$surface and therefore it must be removed by drying to produce an active oxidant.
Complete answer:
Let us first understand the oxidation of allylic and benzylic alcohol with the help of manganese (IV) dioxide:-
-The allylic and benzylic alcohols are oxidized specifically and selectively by a suspension of activated manganese (IV) dioxide ($Mn{{O}_{2}}$). Aldehydes are formed by the oxidation of primary allylic alcohols and ketones are formed by the oxidation of secondary allylic alcohols.
-For this reaction, we require an “activated$Mn{{O}_{2}}$” which is obtained by the oxidation – reduction reaction of potassium permanganate and it is also a mild oxidizing agent. The oxidation of alcohols takes place on the surface of manganese (IV) dioxide ($Mn{{O}_{2}}$) which is insoluble in the solvents used for this reaction.
-The reaction is highly chemoselective because allylic and benzylic alcohols react more rapidly than the ordinary alcohols.
The oxidation reaction of ${{C}_{6}}{{H}_{5}}CH(OH)C{{H}_{2}}C{{H}_{2}}OH$with the help of $Mn{{O}_{2}}$ is shown below:-

We can see that there are two alcohols in the reactant among which $Mn{{O}_{2}}$have selective nature towards benzylic alcohol and hence it will get oxidized to become a carbonyl compound. Since it is a secondary benzylic alcohol, we obtain ketone as a product.
Note:
The other reagents that can be specially used to oxidize allylic and benzylic alcohol are $Mn{{(OAc)}_{2}}$and $Mn{{(OAc)}_{3}}$.
-Remember that water competes with the alcohol for the sites on the $Mn{{O}_{2}}$surface and therefore it must be removed by drying to produce an active oxidant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




