
Give the resonating structures of \[N{{O}_{2}}\]and \[{{N}_{2}}{{O}_{5}}\]
Answer
596.7k+ views
Hint:We know the meaning of resonating structure. From this we have to draw the all delocalization of lone pairs, negative and positive charge etc.
Complete step by step solution:
> We know that resonance is the way by which we describe delocalized electrons within certain molecules or polyatomic ions where the bonding can’t be expressed by a single Lewis formula or structure.
> Resonance structures: These are the set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including partial bonds and partial charges.
> Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
> In case of\[N{{O}_{2}}\],it has one single bonded oxygen and one double bonded oxygen. Or we can say that this double bond delocalized. Below given are resonating structures of \[N{{O}_{2}}\]
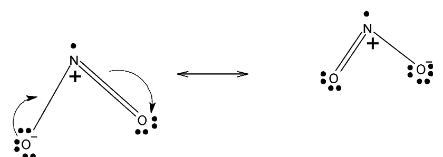
> In case of\[{{N}_{2}}{{O}_{5}}\], it has two double bonded oxygens and one bridge oxygen and two single bonded oxygen. These two double bonds are localized among four oxygens. Below given are resonating structures of \[{{N}_{2}}{{O}_{5}}\]
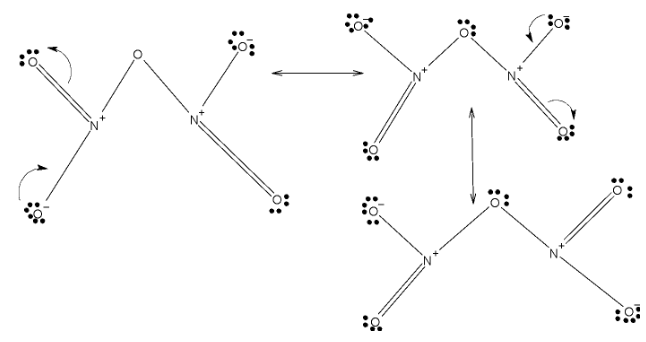
So, the \[N{{O}_{2}}\]has two resonating structures and \[{{N}_{2}}{{O}_{5}}\] has 3 resonating structures
Note: So, while drawing resonating structures you should be careful about lone pairs and charges. Here all double bonds partially exist. And oxygen has partial negative charge.
Complete step by step solution:
> We know that resonance is the way by which we describe delocalized electrons within certain molecules or polyatomic ions where the bonding can’t be expressed by a single Lewis formula or structure.
> Resonance structures: These are the set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including partial bonds and partial charges.
> Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
> In case of\[N{{O}_{2}}\],it has one single bonded oxygen and one double bonded oxygen. Or we can say that this double bond delocalized. Below given are resonating structures of \[N{{O}_{2}}\]

> In case of\[{{N}_{2}}{{O}_{5}}\], it has two double bonded oxygens and one bridge oxygen and two single bonded oxygen. These two double bonds are localized among four oxygens. Below given are resonating structures of \[{{N}_{2}}{{O}_{5}}\]

So, the \[N{{O}_{2}}\]has two resonating structures and \[{{N}_{2}}{{O}_{5}}\] has 3 resonating structures
Note: So, while drawing resonating structures you should be careful about lone pairs and charges. Here all double bonds partially exist. And oxygen has partial negative charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




