
Give the structural formula of phosphorous acid $ ({H_3}P{O_3}) $ and pyrophosphoric acid $ ({H_4}{P_2}{O_7}) $
Answer
497.7k+ views
Hint: Phosphorous acid is the compound described by the formula $ ({H_3}P{O_3}) $ . This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorus acid is an intermediate in the preparation of other phosphorus compounds And Diphosphoric acid is an acyclic phosphorus acid anhydride obtained by condensation of two molecules of phosphoric acid.
Complete Step By Step Answer:
Phosphorous acid:
A phosphorus oxoacid that consists of a single pentavalent phosphorus covalently bound via single bonds to a single hydrogen and two hydroxyl groups and via a double bond to an oxygen. The parent of the class of phosphonic acids. $ {H_3}P{O_3} $ is more clearly described with the structural formula $ HPO{(OH)_2} $ . In the solid state, $ HP(O){(OH)_2} $ is tetrahedral with a $ P - H $ bond of $ 1.32pm $ , one shorter $ P = O $ bond of $ 148pm $ and two longer $ P - O(OH) $ bonds of $ 154pm $ . This species exists in equilibrium with an extremely minor tautomer $ P{(OH)_3} $ .
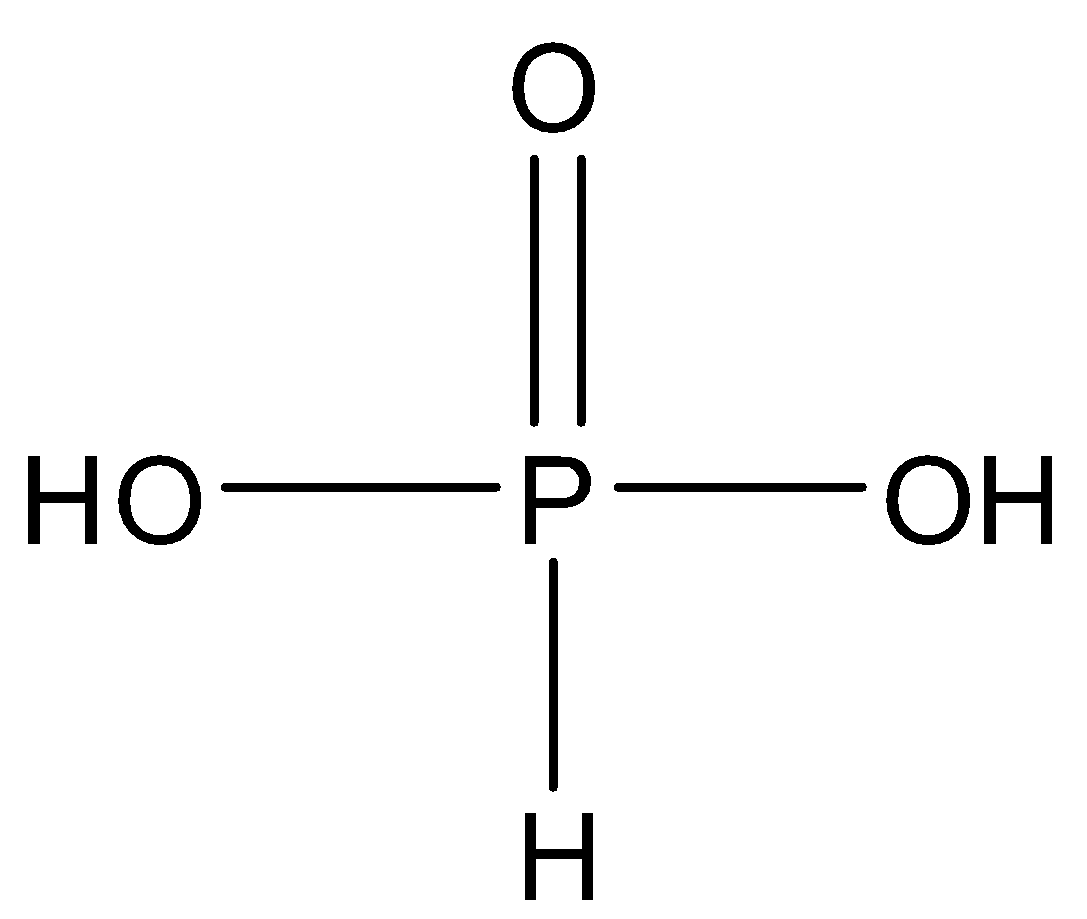
Pyrophosphoric acid:
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula $ {H_4}{P_2}{O_7} $ . Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs.
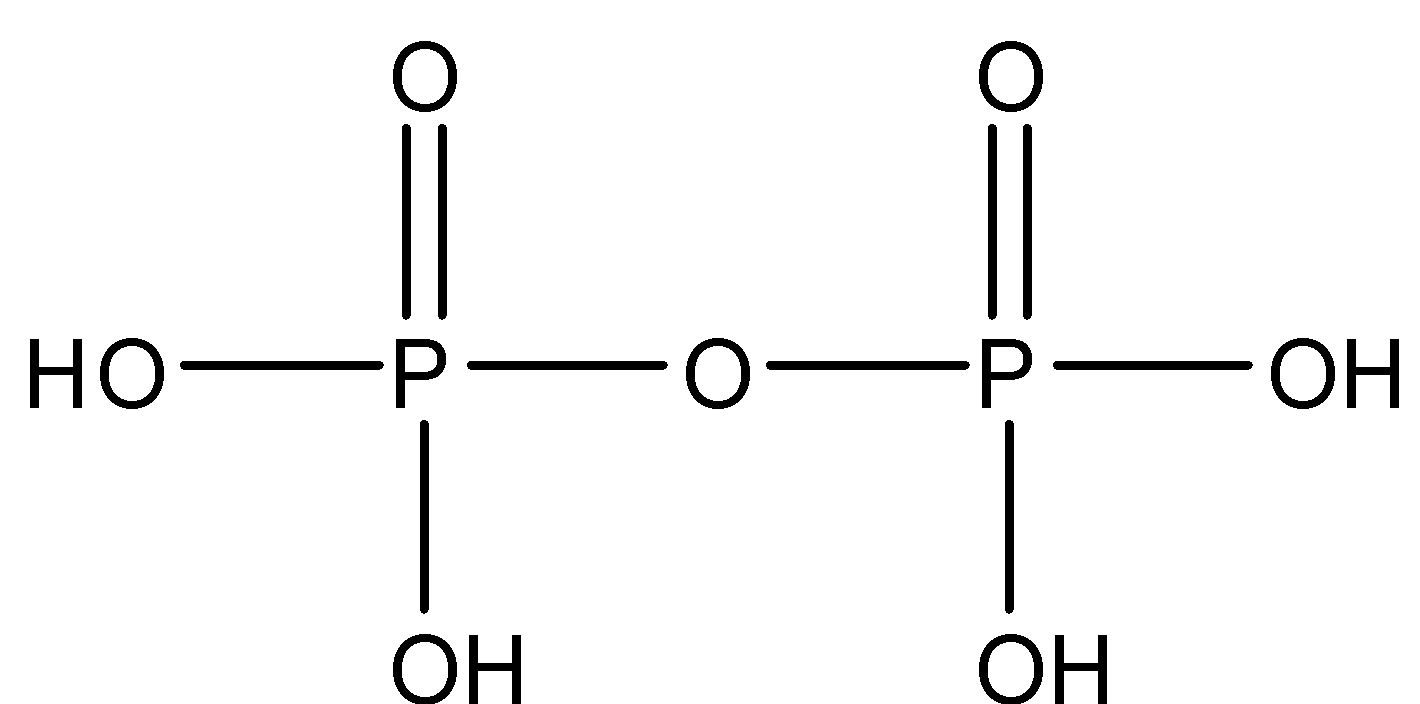
Note:
The most important use of phosphorous acid is the production of basic lead phosphite, which is a stabilizer in $ PVC $ and related chlorinated polymers. It is used in the production of basic lead phosphonate $ PVC $ stabilizer, aminomethylene phosphonic acid and hydroxyethane diphosphonic acid. It is also used as a strong reducing agent and in the production of phosphorus acid, synthetic fibres, organophosphorus pesticides, and the highly efficient water treatment agent. While pyrophosphoric acid is corrosive, it is not known to be otherwise toxic
Complete Step By Step Answer:
Phosphorous acid:
A phosphorus oxoacid that consists of a single pentavalent phosphorus covalently bound via single bonds to a single hydrogen and two hydroxyl groups and via a double bond to an oxygen. The parent of the class of phosphonic acids. $ {H_3}P{O_3} $ is more clearly described with the structural formula $ HPO{(OH)_2} $ . In the solid state, $ HP(O){(OH)_2} $ is tetrahedral with a $ P - H $ bond of $ 1.32pm $ , one shorter $ P = O $ bond of $ 148pm $ and two longer $ P - O(OH) $ bonds of $ 154pm $ . This species exists in equilibrium with an extremely minor tautomer $ P{(OH)_3} $ .

Pyrophosphoric acid:
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula $ {H_4}{P_2}{O_7} $ . Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs.

Note:
The most important use of phosphorous acid is the production of basic lead phosphite, which is a stabilizer in $ PVC $ and related chlorinated polymers. It is used in the production of basic lead phosphonate $ PVC $ stabilizer, aminomethylene phosphonic acid and hydroxyethane diphosphonic acid. It is also used as a strong reducing agent and in the production of phosphorus acid, synthetic fibres, organophosphorus pesticides, and the highly efficient water treatment agent. While pyrophosphoric acid is corrosive, it is not known to be otherwise toxic
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




