
Give the structural formula of the following: Ethanoic acid.
Answer
565.5k+ views
Hint: Acidic corrosive \[\left( {C{H_3}COOH} \right)\],additionally called ethanoic corrosive, the most significant of the carboxylic acids. A weak (around 5 percent by volume) arrangement of acidic corrosive delivered by maturation and oxidation of regular sugars is called vinegar; a salt, ester, or acylal of acidic corrosive is called acetic acid derivation.
Complete step by step answer:
Acidic corrosive is otherwise called ethanoic corrosive, ethylic corrosive, vinegar corrosive, and methane carboxylic corrosive; it has the compound equation of \[\left( {C{H_3}COOH} \right)\]. Acidic corrosive is a result of maturation, and gives vinegar its trademark smell. Vinegar is around \[4 - 6\% \] acidic corrosive in water. Ethanoic corrosive is generally utilized in numerous enterprises. Financially it is utilized in the assembling of esters, vinegar, and numerous polymeric materials. Solid acids separate completely in water to create the greatest number of H + particles. Feeble acids, for example, ethanoic corrosive \[\left( {C{H_3}COOH} \right)\],don't completely separate. Truth is told, about only one percent of ethanoic corrosive particles split up to shape H + particles and \[C{H_3}COO\]– particles at any one time. Ethanoic or acidic corrosive is utilized in making colours, shades, and paint and covering added substances. It is utilized in imprinting on texture. It is a segment of wood stick and different sealants. Acidic corrosive is additionally utilized as a cleaning and degreasing dissolvable.
Structural Formula is: \[\left( {C{H_3}COOH} \right)\]
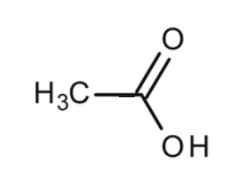
They are made out of three hydrogen ions and one carbon molecule (\[C{H_3}\]). Responses of ethanoic acids:
Esterification response:
At the point when carboxylic corrosive and liquor respond, the item framed is known as an ester. The following is an illustration of the arrangement of an ester from the response of ethanoic corrosive with supreme ethanol within the sight of a corrosive as an impetus.
\[C{H_3}COOH\;\;\;\;{\text{ }} + \;\;\;\;\;{\text{ }}C{H_3}C{H_2}OH{\text{ }}\;{\text{ }}\;{\text{ }}\; \to \;\;{\text{ }}\;{\text{ }}C{H_3}COOC{H_2}C{H_3}\]
$\text{(Ethanoic corrosive) (Ethanol) (Esters)}$
Esters have a sweet fruity smell. They are essentially utilized for making fragrances and manufactured enhancing specialists. The response of esters with soluble bases gives carboxylic corrosive salt and liquor. This response is utilized in the creation of cleansers and the cycle is called a saponification response.
\[C{H_3}COO{C_2}{H_{5\;\;\;\;\;\;}} + {\text{ }}NaOH\;\;\;\;\;\; \to \;\;{\text{ }}\;{\text{ }}\;{\text{ }}\;{\text{ }}{C_2}{H_5}OH{\text{ }} + \;{\text{ }}C{H_3}COONa\]
Response with a base:
Ethanoic corrosive responds with a base to give the salt and water simply like other mineral acids.
Response with carbonates and hydrogen carbonates:
Carbon dioxide, salt, and water are created when ethanoic corrosive responds with carbonates and hydrogen carbonates. Sodium acetic acid derivation is generally created as a salt when ethanoic corrosive responds with sodium bicarbonate as appeared in the response underneath:
\[C{H_3}COOH\;{\text{ }} + \;{\text{ }}NaHC{O_3}\;{\text{ }}\;{\text{ }}\; \to \;\;{\text{ }}\;{\text{ }}\;C{H_3}COONa{\text{ }} + {\text{ }}{H_2}O{\text{ }} + {\text{ }}C{O_2}\]
Note: Ethanoic corrosive is generally utilized in numerous enterprises.
Industrially it is utilized in the assembling of esters, vinegar, and numerous polymeric materials.
Vinegar has appeared to lessen high groupings of glucose.
Utilized as a specialist to lyses red platelets before white platelets are analyzed.
Utilized as a dissolvable in the creation of camphor, rising and cooking fixing.
Complete step by step answer:
Acidic corrosive is otherwise called ethanoic corrosive, ethylic corrosive, vinegar corrosive, and methane carboxylic corrosive; it has the compound equation of \[\left( {C{H_3}COOH} \right)\]. Acidic corrosive is a result of maturation, and gives vinegar its trademark smell. Vinegar is around \[4 - 6\% \] acidic corrosive in water. Ethanoic corrosive is generally utilized in numerous enterprises. Financially it is utilized in the assembling of esters, vinegar, and numerous polymeric materials. Solid acids separate completely in water to create the greatest number of H + particles. Feeble acids, for example, ethanoic corrosive \[\left( {C{H_3}COOH} \right)\],don't completely separate. Truth is told, about only one percent of ethanoic corrosive particles split up to shape H + particles and \[C{H_3}COO\]– particles at any one time. Ethanoic or acidic corrosive is utilized in making colours, shades, and paint and covering added substances. It is utilized in imprinting on texture. It is a segment of wood stick and different sealants. Acidic corrosive is additionally utilized as a cleaning and degreasing dissolvable.
Structural Formula is: \[\left( {C{H_3}COOH} \right)\]

They are made out of three hydrogen ions and one carbon molecule (\[C{H_3}\]). Responses of ethanoic acids:
Esterification response:
At the point when carboxylic corrosive and liquor respond, the item framed is known as an ester. The following is an illustration of the arrangement of an ester from the response of ethanoic corrosive with supreme ethanol within the sight of a corrosive as an impetus.
\[C{H_3}COOH\;\;\;\;{\text{ }} + \;\;\;\;\;{\text{ }}C{H_3}C{H_2}OH{\text{ }}\;{\text{ }}\;{\text{ }}\; \to \;\;{\text{ }}\;{\text{ }}C{H_3}COOC{H_2}C{H_3}\]
$\text{(Ethanoic corrosive) (Ethanol) (Esters)}$
Esters have a sweet fruity smell. They are essentially utilized for making fragrances and manufactured enhancing specialists. The response of esters with soluble bases gives carboxylic corrosive salt and liquor. This response is utilized in the creation of cleansers and the cycle is called a saponification response.
\[C{H_3}COO{C_2}{H_{5\;\;\;\;\;\;}} + {\text{ }}NaOH\;\;\;\;\;\; \to \;\;{\text{ }}\;{\text{ }}\;{\text{ }}\;{\text{ }}{C_2}{H_5}OH{\text{ }} + \;{\text{ }}C{H_3}COONa\]
Response with a base:
Ethanoic corrosive responds with a base to give the salt and water simply like other mineral acids.
Response with carbonates and hydrogen carbonates:
Carbon dioxide, salt, and water are created when ethanoic corrosive responds with carbonates and hydrogen carbonates. Sodium acetic acid derivation is generally created as a salt when ethanoic corrosive responds with sodium bicarbonate as appeared in the response underneath:
\[C{H_3}COOH\;{\text{ }} + \;{\text{ }}NaHC{O_3}\;{\text{ }}\;{\text{ }}\; \to \;\;{\text{ }}\;{\text{ }}\;C{H_3}COONa{\text{ }} + {\text{ }}{H_2}O{\text{ }} + {\text{ }}C{O_2}\]
Note: Ethanoic corrosive is generally utilized in numerous enterprises.
Industrially it is utilized in the assembling of esters, vinegar, and numerous polymeric materials.
Vinegar has appeared to lessen high groupings of glucose.
Utilized as a specialist to lyses red platelets before white platelets are analyzed.
Utilized as a dissolvable in the creation of camphor, rising and cooking fixing.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




