
Give the structure and IUPAC names of the products expected from the following reaction:
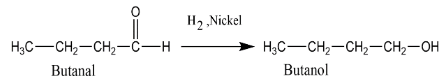
A. Catalytic reduction of butanal.
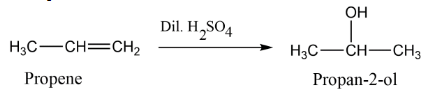
B. Hydration of propene in the presence of dilute sulphuric acid.
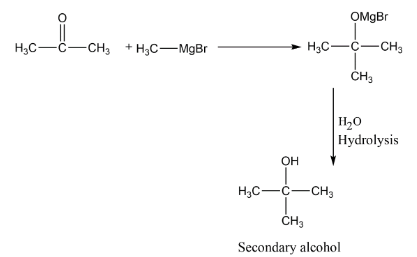
C. Reaction of propanone with methylmagnesium bromide followed by hydrolysis.
Answer
546.6k+ views
Hint: Try to write the process according to the chemical reaction happening in the presence of that reagent, there are some oxidizing agents which oxidize the group and also there are some reducing agents. The most commonly used reagent for reduction is hydrogen with nickel, nickel is the catalyst there. Always remember that on reduction of aldehyde we will get alcohol.
Complete step-by-step answer:
A. Catalytic reduction is the reduction in which reduction is taking place but in presence of some catalysis. We have butanal which is an aldehyde, for reduction we are using hydrogen in the presence of nickel metal \[H{}_2,Ni\]. Here nickel metal is a catalyst. On reducing butanal, it changes to butanol which is an alcohol.
B. Next we have propene, it means we have a three carbon compound with a double bond it is an unsaturated hydrocarbon. Now we have to do the hydrolysis of propene in the presence of dilute sulphuric acid $dil.\,{H_2}S{O_4}$. Let’s see by reaction that the double bond gets converted into a single bond and an alcoholic group gets introduced to the carbon atom having lesser number of hydrogen around double bond. Thus the addition takes place according to Markonikov rule.
C. We have Grignard reagent as methylmagnesium bromide $C{H_3} - MgBr$ and we have to add this reagent to propanone which is a ketone. Let’s see with the help of reaction that in the first step, the negative part of reagent which is methyl anion will attack on electrophilic carbon of propanone, then after hydrolysis we will get the secondary alcohols.
Note: In option C, during hydrolysis the bond between oxygen and magnesium will cleave such that oxygen will get hydrogen and magnesium will get from water molecules on hydrolysis. In option B, when hydrolysis of propene happens, it will occur according to the Markonikov rule of addition while in some reactions, addition also takes place according to Anti-Markovnikov rule when presence of peroxide will be given.
Complete step-by-step answer:
A. Catalytic reduction is the reduction in which reduction is taking place but in presence of some catalysis. We have butanal which is an aldehyde, for reduction we are using hydrogen in the presence of nickel metal \[H{}_2,Ni\]. Here nickel metal is a catalyst. On reducing butanal, it changes to butanol which is an alcohol.

B. Next we have propene, it means we have a three carbon compound with a double bond it is an unsaturated hydrocarbon. Now we have to do the hydrolysis of propene in the presence of dilute sulphuric acid $dil.\,{H_2}S{O_4}$. Let’s see by reaction that the double bond gets converted into a single bond and an alcoholic group gets introduced to the carbon atom having lesser number of hydrogen around double bond. Thus the addition takes place according to Markonikov rule.

C. We have Grignard reagent as methylmagnesium bromide $C{H_3} - MgBr$ and we have to add this reagent to propanone which is a ketone. Let’s see with the help of reaction that in the first step, the negative part of reagent which is methyl anion will attack on electrophilic carbon of propanone, then after hydrolysis we will get the secondary alcohols.

Note: In option C, during hydrolysis the bond between oxygen and magnesium will cleave such that oxygen will get hydrogen and magnesium will get from water molecules on hydrolysis. In option B, when hydrolysis of propene happens, it will occur according to the Markonikov rule of addition while in some reactions, addition also takes place according to Anti-Markovnikov rule when presence of peroxide will be given.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




