
Given are two figures of Daniell cell (X) and (Y). Study the figures and mark the incorrect statement from the following.
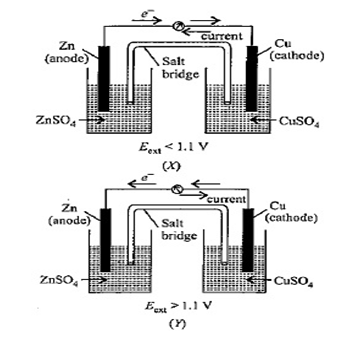
(A)- In fig(X), electrons flow from Zn rod to Cu rod hence current flows from Cu to Zn (${{E}_{ext}}$< 1.1V)
(B)- In fig(Y), electrons flow from Cu to Zn and current flows from Zn to Cu (${{E}_{ext}}$ >1.1V)
(C)- In fig (X), Zn dissolves at anode and Cu deposits at cathode.
(D)- In fig (Y), Zn is deposited at Cu and Cu is deposited at Zn.

Answer
569.7k+ views
Hint: Oxidation (loss of electrons) occurs at anode and reduction (gain of electrons) occurs at cathode.
When an opposing external potential is applied to a cell becomes more than the electric potential of the cell, the redox reaction occurs in the opposite direction.
Complete Solution :
Daniell cell is an electrochemical cell and has an electrical potential of 1.1V. It converts the chemical energy of the given redox reaction into electrical energy.
\[Zn(s)+C{{u}^{2+}}(aq)\to Z{{n}^{2+}}+Cu(s)\]
When ${{E}_{ext}}$ < 1.1 V
Oxidation occurs at Zn electrode and reduction occurs at Cu electrode.
Electrons from the Zn electrode (anode) flow toward Cu electrode (cathode) in the external circuit.
However, the direction of current is opposite to the direction of flow of electrons, i.e. from Cu to Zn electrode.
Zn oxidized into $Z{{n}^{2+}}$ ions at anode and $C{{u}^{2+}}$ ions reduce to Cu atoms at cathode. In other words, Zn dissolves at anode and Cu deposits at cathode.
When ${{E}_{ext}}$ > 1.1 V
- When an opposing external potential is applied to a cell, the reaction continues to occur in the same direction until ${{E}_{ext}}$ becomes equal to the cell potential. When ${{E}_{cell}}={{E}_{ext}}=1.1$V, no chemical reaction occurs and flow of electrons stops. If ${{E}_{ext}}$ further continues to increase, the reactions start in opposite reactions such that Electrons flow from Cu electrode to Zn electrode in the external circuit.
- Current flows in the direction opposite to that of electrons, i.e. from Zn to Cu electrode.
- Cu from Cu electrode dissolves into solution and Zn gets deposited at Zn electrode.
- Now the electric energy is used to bring about the otherwise non-spontaneous ($\Delta G$ is positive) chemical reaction. Hence, Daneill cell now behaves as an electrolytic cell.
From the above discussion, we conclude that Zn is never deposited at Cu and vice-versa. So, it is an incorrect statement.
So, the correct answer is “Option D”.
Additional information: Salt bridge allows the movement of ions from one solution to another without mixing the two solutions to complete the inner circuit. It helps to maintain the electrical neutrality of the solution. For instance, excess of $Z{{n}^{2+}}$ ions produced from oxidation and excess of $SO_{4}^{-2}$ ions left due to the reduction of $C{{u}^{2+}}$ from the solution into Cu, move through the salt bridge to maintain the electrical neutrality of the solution. This completes the inner circuit of the cell.
Note: Daniell cell works as a galvanic cell when ${{E}_{ext}}$ < 1.1 V and as an electrolytic cell when ${{E}_{ext}}$ > 1.1 V. Galvanic cell converts the chemical energy produced as a result of redox reaction into electrical energy whereas an electrolytic cell converts the electrical energy into chemical energy.
When an opposing external potential is applied to a cell becomes more than the electric potential of the cell, the redox reaction occurs in the opposite direction.
Complete Solution :
Daniell cell is an electrochemical cell and has an electrical potential of 1.1V. It converts the chemical energy of the given redox reaction into electrical energy.
\[Zn(s)+C{{u}^{2+}}(aq)\to Z{{n}^{2+}}+Cu(s)\]
When ${{E}_{ext}}$ < 1.1 V
Oxidation occurs at Zn electrode and reduction occurs at Cu electrode.
Electrons from the Zn electrode (anode) flow toward Cu electrode (cathode) in the external circuit.
However, the direction of current is opposite to the direction of flow of electrons, i.e. from Cu to Zn electrode.
Zn oxidized into $Z{{n}^{2+}}$ ions at anode and $C{{u}^{2+}}$ ions reduce to Cu atoms at cathode. In other words, Zn dissolves at anode and Cu deposits at cathode.
When ${{E}_{ext}}$ > 1.1 V
- When an opposing external potential is applied to a cell, the reaction continues to occur in the same direction until ${{E}_{ext}}$ becomes equal to the cell potential. When ${{E}_{cell}}={{E}_{ext}}=1.1$V, no chemical reaction occurs and flow of electrons stops. If ${{E}_{ext}}$ further continues to increase, the reactions start in opposite reactions such that Electrons flow from Cu electrode to Zn electrode in the external circuit.
- Current flows in the direction opposite to that of electrons, i.e. from Zn to Cu electrode.
- Cu from Cu electrode dissolves into solution and Zn gets deposited at Zn electrode.
- Now the electric energy is used to bring about the otherwise non-spontaneous ($\Delta G$ is positive) chemical reaction. Hence, Daneill cell now behaves as an electrolytic cell.
From the above discussion, we conclude that Zn is never deposited at Cu and vice-versa. So, it is an incorrect statement.
So, the correct answer is “Option D”.
Additional information: Salt bridge allows the movement of ions from one solution to another without mixing the two solutions to complete the inner circuit. It helps to maintain the electrical neutrality of the solution. For instance, excess of $Z{{n}^{2+}}$ ions produced from oxidation and excess of $SO_{4}^{-2}$ ions left due to the reduction of $C{{u}^{2+}}$ from the solution into Cu, move through the salt bridge to maintain the electrical neutrality of the solution. This completes the inner circuit of the cell.
Note: Daniell cell works as a galvanic cell when ${{E}_{ext}}$ < 1.1 V and as an electrolytic cell when ${{E}_{ext}}$ > 1.1 V. Galvanic cell converts the chemical energy produced as a result of redox reaction into electrical energy whereas an electrolytic cell converts the electrical energy into chemical energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




