
Glucose and mannose are:
A. anomers
B. epimers
C. ketohexoses
D. Disaccharides
Answer
592.5k+ views
Hint: Glucose and mannose are the type of stereoisomers in which the compounds differ from each other in configuration at the stereogenic centers. They have the same chemical formula.
Complete answer:
Glucose and mannose have the same chemical formula, that is ${C}_{6}{H}_{12}{O}_{6}$. The molecular structure of glucose and mannose respectively are:
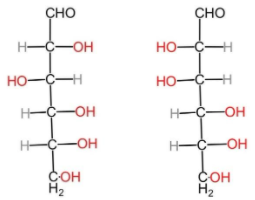
Glucose $\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$ Mannose
Let us now look at each term given in the question.
(A) Anomers: Anomers are the diastereoisomers of cyclic sugars or similar molecules that differ in the configuration at the anomeric carbon. Now, since, glucose and mannose are not cyclic compounds, therefore, they are not anomers.
(B) Epimers: Epimers are the compounds in which the isomers have different configurations of atoms about one of the several asymmetrical carbon atoms present. They are a specific type of stereoisomers which have multiple stereocenters but differ from one another by the configuration of one of the stereogenic centers. In case of glucose and mannose, they differ from each other by configuration at the C-2 atom. And thus, they are epimers.
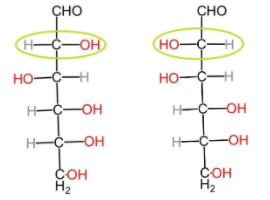
Glucose $\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$ Mannose
(C) Ketohexoses: As the name suggests, ketohexose is a hexose that contains ketone. It is a monosaccharide that has both a ketone and six carbons. But, glucose and mannose do not contain any ketone group in its structure. Therefore, they are not ketohexoses.
(D) Disaccharides: Disaccharides is the sugar that is formed when two monosaccharides are joined by glycosidic linkage. But we can see that glucose and mannose and single compounds. Therefore, they are not disaccharides.
Therefore, glucose and mannose are epimers.
Hence, the correct answer is option (B).
Note: Glucose and mannose are also known as aldohexoses. This is because they contain an aldehyde group. These occur naturally as monosaccharides in fruits and in glycoproteins of algae and fungi.
Complete answer:
Glucose and mannose have the same chemical formula, that is ${C}_{6}{H}_{12}{O}_{6}$. The molecular structure of glucose and mannose respectively are:

Glucose $\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$ Mannose
Let us now look at each term given in the question.
(A) Anomers: Anomers are the diastereoisomers of cyclic sugars or similar molecules that differ in the configuration at the anomeric carbon. Now, since, glucose and mannose are not cyclic compounds, therefore, they are not anomers.
(B) Epimers: Epimers are the compounds in which the isomers have different configurations of atoms about one of the several asymmetrical carbon atoms present. They are a specific type of stereoisomers which have multiple stereocenters but differ from one another by the configuration of one of the stereogenic centers. In case of glucose and mannose, they differ from each other by configuration at the C-2 atom. And thus, they are epimers.

Glucose $\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$ Mannose
(C) Ketohexoses: As the name suggests, ketohexose is a hexose that contains ketone. It is a monosaccharide that has both a ketone and six carbons. But, glucose and mannose do not contain any ketone group in its structure. Therefore, they are not ketohexoses.
(D) Disaccharides: Disaccharides is the sugar that is formed when two monosaccharides are joined by glycosidic linkage. But we can see that glucose and mannose and single compounds. Therefore, they are not disaccharides.
Therefore, glucose and mannose are epimers.
Hence, the correct answer is option (B).
Note: Glucose and mannose are also known as aldohexoses. This is because they contain an aldehyde group. These occur naturally as monosaccharides in fruits and in glycoproteins of algae and fungi.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




