
Glucose reacts with bromine water to produce:
A. glyceraldehyde
B. gluconic acid
C. saccharic acid
D. glucaric acid
Answer
586.2k+ views
Hint: We know that bromine water is a mild oxidizing agent. It only oxidizes aldehydic group of glucose to carboxylic acid. The other \[ - {\rm{OH}}\] in the chain remains unaffected. It is also known that a glucose molecule contains 6 membered chain with only one aldehydic group.
Complete step by step answer:
We know that glucose has molecular formula \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\]. It is a monosaccharide that contains six carbon atoms along with one \[{\rm{CHO}}\] group. So when bromine water which is a mild oxidizing agent is added in glucose, that \[{\rm{CHO}}\] group will get oxidized to \[{\rm{COOH}}\]. The product formed is gluconic acid.
We can write this chemical change as:
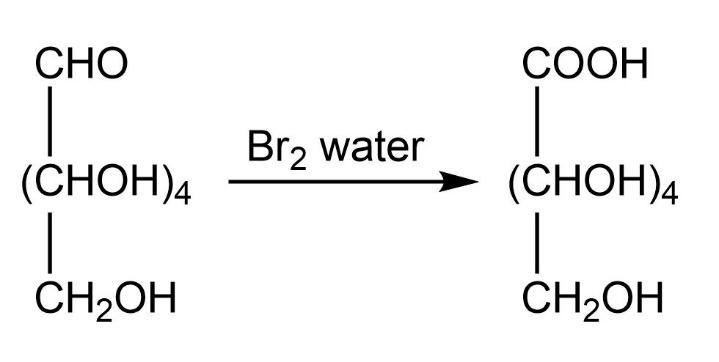
So, the correct answer is “Option B”.
Note:
We know that aldehydes are very prone to oxidizing agents. They get easily oxidized to carboxylic acids with any mild oxidizing agent. Also glucose has only one aldehydic group and addition of this oxidizing agent converts this group into \[{\rm{COOH}}\].
Complete step by step answer:
We know that glucose has molecular formula \[{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}\]. It is a monosaccharide that contains six carbon atoms along with one \[{\rm{CHO}}\] group. So when bromine water which is a mild oxidizing agent is added in glucose, that \[{\rm{CHO}}\] group will get oxidized to \[{\rm{COOH}}\]. The product formed is gluconic acid.
We can write this chemical change as:

So, the correct answer is “Option B”.
Note:
We know that aldehydes are very prone to oxidizing agents. They get easily oxidized to carboxylic acids with any mild oxidizing agent. Also glucose has only one aldehydic group and addition of this oxidizing agent converts this group into \[{\rm{COOH}}\].
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




