
What group in the periodic table is nitrogen located?
Answer
524.1k+ views
Hint: The modern periodic table is the arrangement of the elements according to the increasing order of their atomic numbers. The periodic table consists of elements distributed into various periods and groups. The horizontal row is the period and the vertical columns are the groups. There are 7 periods and 18 groups in the modern periodic table.
Complete answer:
The modern periodic table is made on the basis of modern periodic law that states, the physical and chemical properties of elements are the periodic function of their atomic number. This results in the arrangement of elements in the periodic table according to the increasing atomic number. The atomic number of elements is the total number of protons which is also equal to the total number of electrons in that atom.
A modern periodic table consists of 18 groups and 7 periods. Groups are vertical columns and periods are horizontal rows. The periodic table is as follows:
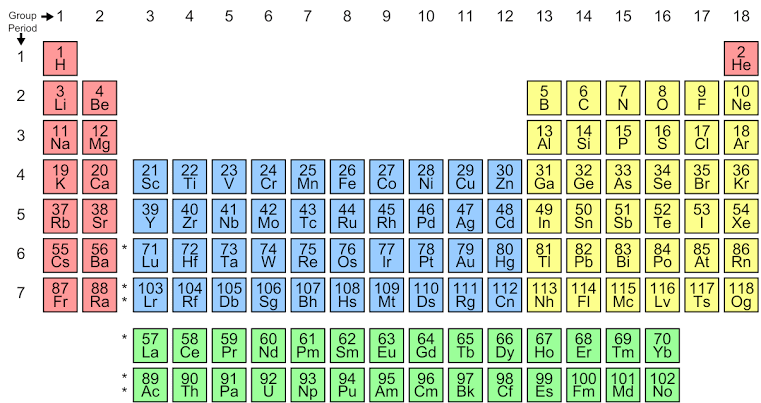
Clearly, through this table, the position of nitrogen with its symbol N is in group 15.
Hence, nitrogen is located in the group 15 of the periodic table.
Note:
According to the periodic table of Mendeleev, the position of nitrogen is in group VA. The modern periodic table is based on his arrangement of the periodic table. The group 15 are called as pnictogens, that consist of the non – metals that are nitrogen (N) and phosphorus (P), while arsenic (As) and antimony (Sb) are metalloids and bismuth (Bi) is a metal.
Complete answer:
The modern periodic table is made on the basis of modern periodic law that states, the physical and chemical properties of elements are the periodic function of their atomic number. This results in the arrangement of elements in the periodic table according to the increasing atomic number. The atomic number of elements is the total number of protons which is also equal to the total number of electrons in that atom.
A modern periodic table consists of 18 groups and 7 periods. Groups are vertical columns and periods are horizontal rows. The periodic table is as follows:

Clearly, through this table, the position of nitrogen with its symbol N is in group 15.
Hence, nitrogen is located in the group 15 of the periodic table.
Note:
According to the periodic table of Mendeleev, the position of nitrogen is in group VA. The modern periodic table is based on his arrangement of the periodic table. The group 15 are called as pnictogens, that consist of the non – metals that are nitrogen (N) and phosphorus (P), while arsenic (As) and antimony (Sb) are metalloids and bismuth (Bi) is a metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




