
What happens when aniline reacts with the following:-
Nitrous acid
Answer
517.2k+ views
Hint: As we know that aniline is an organic compound that consists of a phenyl group attached to an amino group. It is the simplest aromatic amine available. It is industrially very significant and used as a starting reaction in organic synthesis of various compounds. Here we have to tell the product formed when aniline reacts with nitrous acid.
Complete answer:
Let us discuss about reactive nature of aniline followed by the reaction as follows:-
Just like phenols, aniline does give electrophilic substitution reaction. Due to its high reactivity, it can also be categorized as an enamine. When aniline or its ring-substituted derivatives react with nitrous acid, they form diazonium salts or ions. With the help of this intermediate, aniline can be conveniently converted to phenol or aryl halide. The reaction takes place as follows:-
(a)Formation of diazonium ion:-
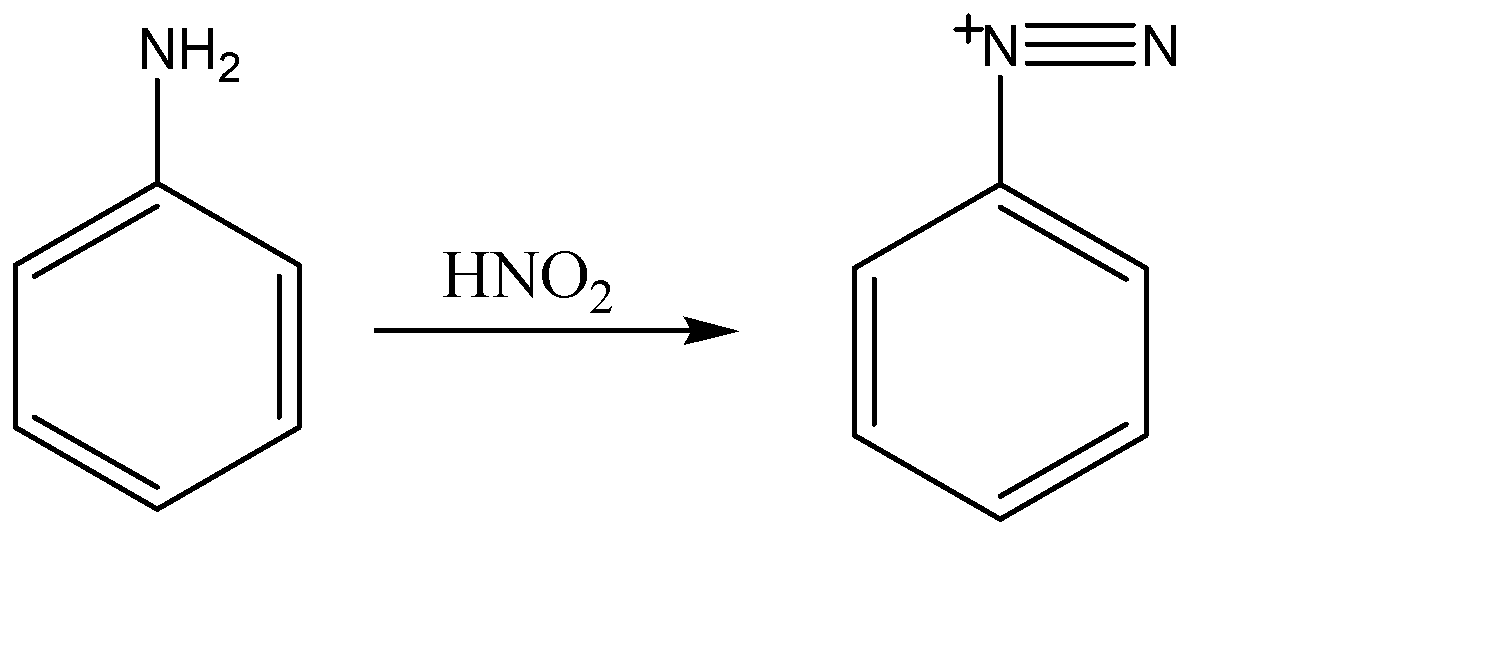
When nitric acid ($HN{{O}_{2}}$) reacts with aniline, the nitrosonium ion of nitric acid attacks amine part of the reactant and converts it into diazonium ion.
-Removal of Nitrogen gas:-
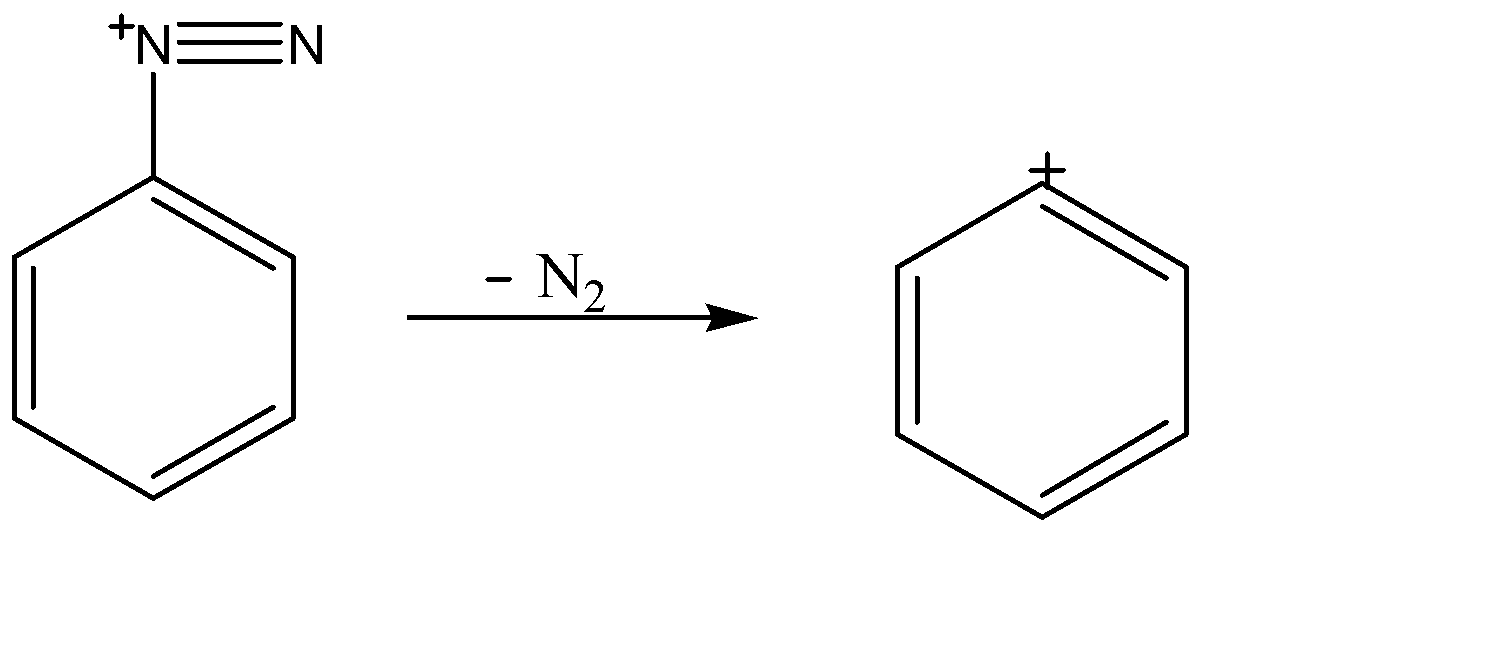
This nitrogen carrying positive charge weakens the C-N bond because it is an electronegative element and cannot tolerate positive charge for a long time. Therefore it decomposes itself into nitrogen gas and a carbocation on the benzene ring.
-Attack of water molecule:-
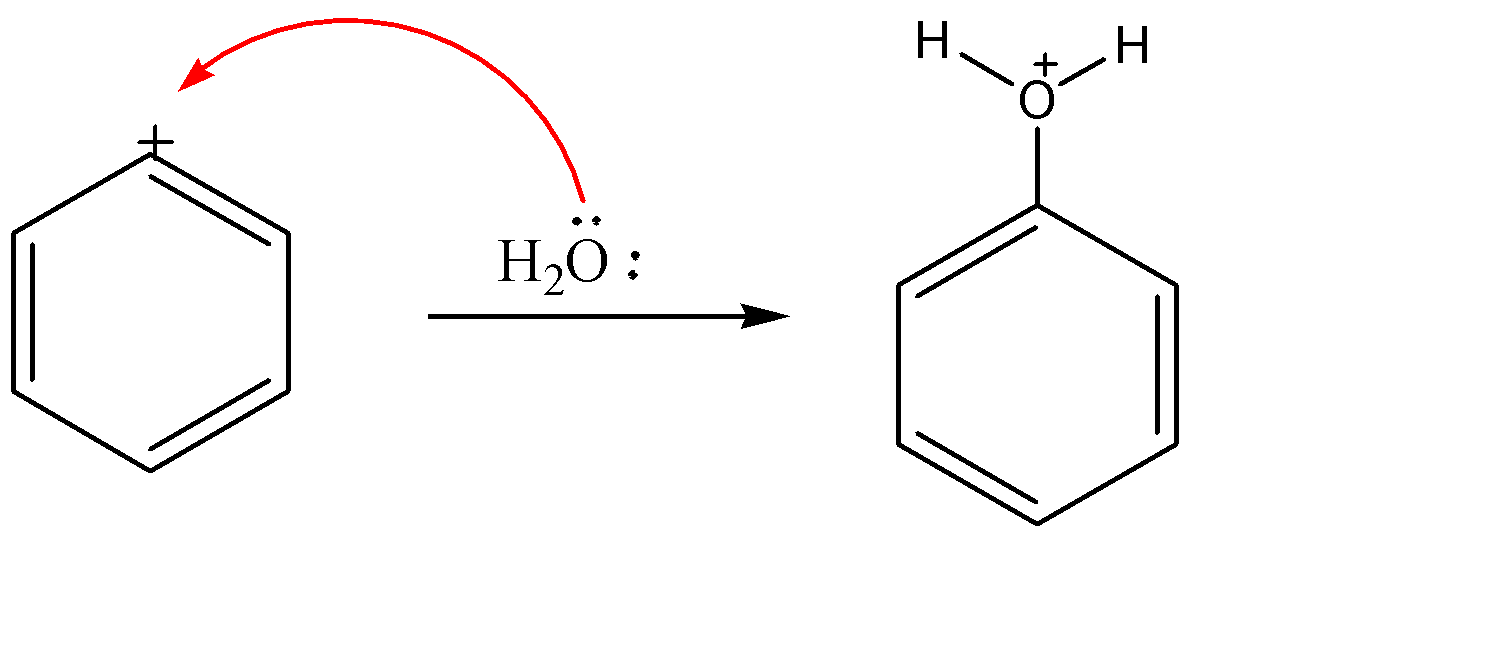
Since benzene is a stable molecule and do not want any charge on it, so it becomes highly reactive towards any nucleophile. When a water molecule arrives, it attacks on carbocation part of the ring which acts as electrophile.
-Formation of phenol:-
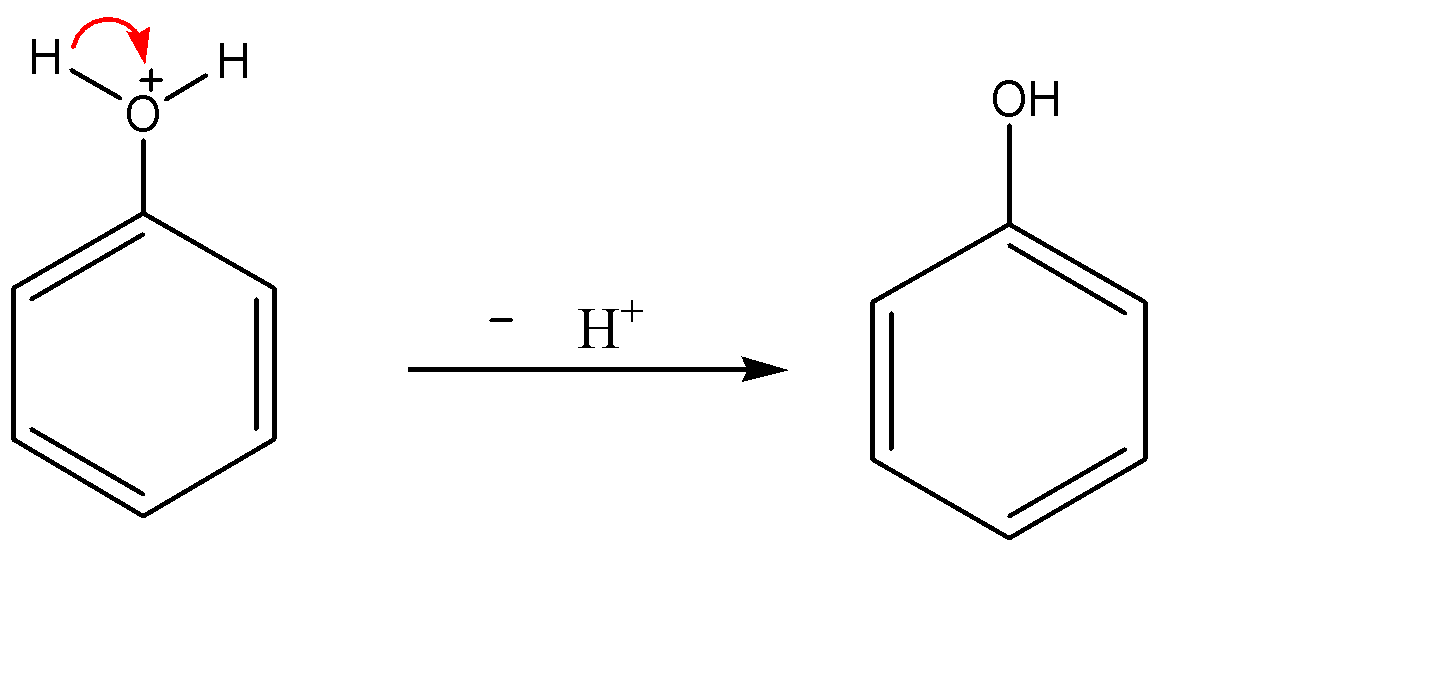
Since oxygen is the second most electronegative element in the periodic table, it would not carry positive charge on itself. Now the electron density of the O-H bond shifts towards the oxygen atom and hydrogen leaves as ${{H}^{+}}$ ion. So we obtain phenol as the product.
-Therefore, when aniline reacts with nitrous acid, it yields phenol in the presence of water.
Note:
-Remember that diazonium salt or ion can also be formed by reacting aniline with sodium nitrate ($NaN{{O}_{2}}$) along with hydrochloric acid. This reaction also takes place at low temperature as well.
-This whole process of converting aniline to diazonium salt or ion is known as diazotization.
Complete answer:
Let us discuss about reactive nature of aniline followed by the reaction as follows:-
Just like phenols, aniline does give electrophilic substitution reaction. Due to its high reactivity, it can also be categorized as an enamine. When aniline or its ring-substituted derivatives react with nitrous acid, they form diazonium salts or ions. With the help of this intermediate, aniline can be conveniently converted to phenol or aryl halide. The reaction takes place as follows:-
(a)Formation of diazonium ion:-

When nitric acid ($HN{{O}_{2}}$) reacts with aniline, the nitrosonium ion of nitric acid attacks amine part of the reactant and converts it into diazonium ion.
-Removal of Nitrogen gas:-

This nitrogen carrying positive charge weakens the C-N bond because it is an electronegative element and cannot tolerate positive charge for a long time. Therefore it decomposes itself into nitrogen gas and a carbocation on the benzene ring.
-Attack of water molecule:-

Since benzene is a stable molecule and do not want any charge on it, so it becomes highly reactive towards any nucleophile. When a water molecule arrives, it attacks on carbocation part of the ring which acts as electrophile.
-Formation of phenol:-

Since oxygen is the second most electronegative element in the periodic table, it would not carry positive charge on itself. Now the electron density of the O-H bond shifts towards the oxygen atom and hydrogen leaves as ${{H}^{+}}$ ion. So we obtain phenol as the product.
-Therefore, when aniline reacts with nitrous acid, it yields phenol in the presence of water.
Note:
-Remember that diazonium salt or ion can also be formed by reacting aniline with sodium nitrate ($NaN{{O}_{2}}$) along with hydrochloric acid. This reaction also takes place at low temperature as well.
-This whole process of converting aniline to diazonium salt or ion is known as diazotization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




