
What happens when but-2-yne is treated with Na in liquid ammonia?
A.Cis-2-butene is formed
B.Trans-2-butene is formed
C.n-butane is the major product
D.It rearranges to but-1-yne
E.There is no reaction
Answer
577.8k+ views
Hint: Sodium in liquid ammonia reduces alkynes to alkenes. The reaction is called Birch reduction. In other words, it hydrogenates the alkynes to form alkenes.
Complete step by step answer:
The reduction of alkynes to alkenes is done with the help of sodium in liquid ammonia. This catalyst readily gives the trans-2-Butene (E-2-Butene). This reaction is known as Birch reduction. In order to obtain cis-2-Butene, Lindlar catalyst can be employed. Lindlar catalyst is a combination of Palladium, lead acetate and quinoline with hydrogen gas being pumped at high pressure.
But-2-yne when treated with sodium in liquid ammonia gives trans-2-butene.
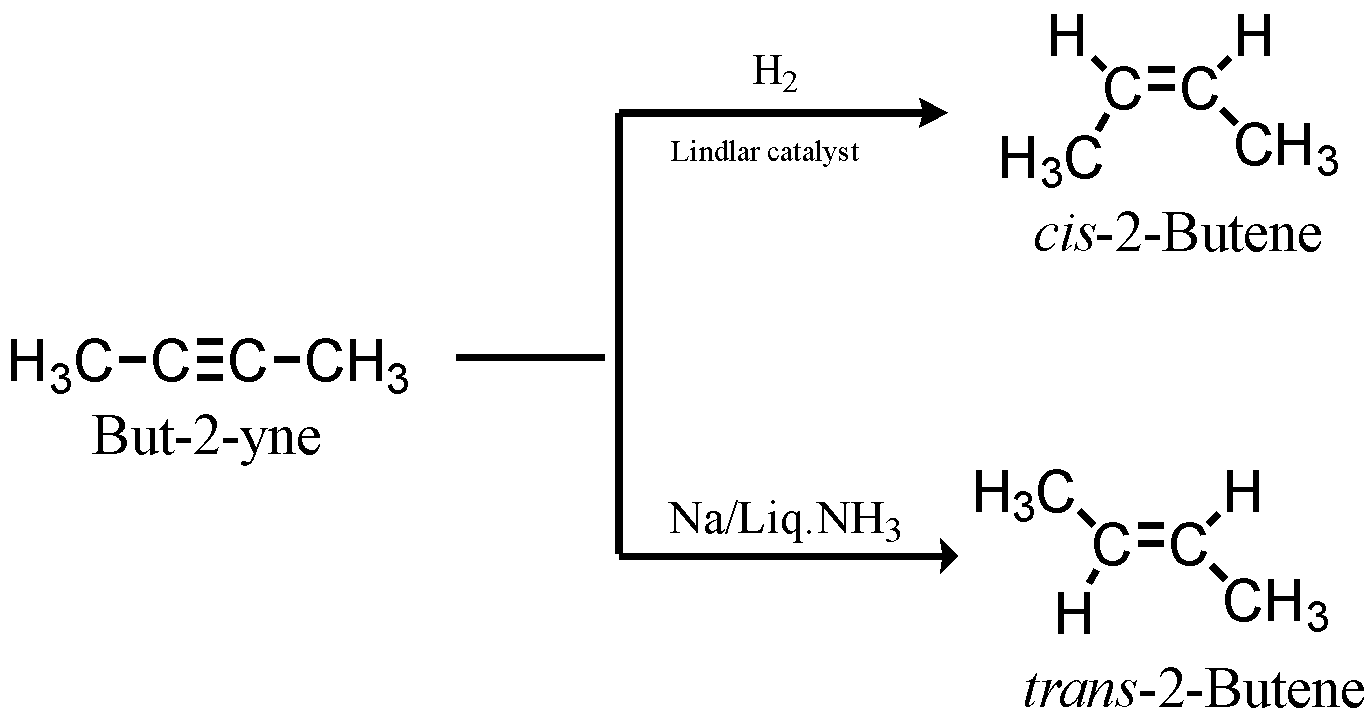
The reaction of alkyne with sodium in liquid ammonia produces sodamide in the process converting alkynes to alkenes.
Mechanism of Birch Reduction:
-Sodium in liquid ammonia produces solvated electrons.
-The attack of solvated electrons forms a radical anion.
-Now, protonation of the radical anion takes place.
-The reduction of radicals to an anion by electrons occurs.
-Now through protonation of the anion by alcohol, desired alkene is formed.
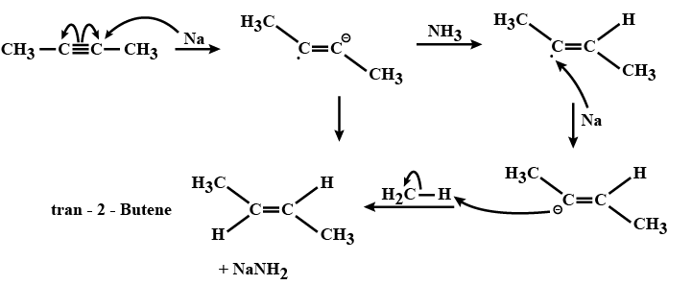
So, the correct option is B.
Note:
Instead of sodium metal, even lithium (Li) metal can be used. Keep in mind sodium amide or with liq. Ammonia is not a Birch reduction. Since sodamide is a strong base and not a reducing agent.
Complete step by step answer:
The reduction of alkynes to alkenes is done with the help of sodium in liquid ammonia. This catalyst readily gives the trans-2-Butene (E-2-Butene). This reaction is known as Birch reduction. In order to obtain cis-2-Butene, Lindlar catalyst can be employed. Lindlar catalyst is a combination of Palladium, lead acetate and quinoline with hydrogen gas being pumped at high pressure.
But-2-yne when treated with sodium in liquid ammonia gives trans-2-butene.

The reaction of alkyne with sodium in liquid ammonia produces sodamide in the process converting alkynes to alkenes.
Mechanism of Birch Reduction:
-Sodium in liquid ammonia produces solvated electrons.
-The attack of solvated electrons forms a radical anion.
-Now, protonation of the radical anion takes place.
-The reduction of radicals to an anion by electrons occurs.
-Now through protonation of the anion by alcohol, desired alkene is formed.

So, the correct option is B.
Note:
Instead of sodium metal, even lithium (Li) metal can be used. Keep in mind sodium amide or with liq. Ammonia is not a Birch reduction. Since sodamide is a strong base and not a reducing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




