
What happens when Phenol reacts with NaOH?
Answer
507.6k+ views
Hint: Phenol has chemical formula ${C_6}{H_5}OH$ and its aqueous solution is weakly acidic in nature while NaOH (sodium hydroxide) is a strong base and hence, the reaction between phenol and sodium hydroxide is an acid-base reaction. It is also known as the neutralization reaction and hence salt and water will be formed.
Complete answer:
Phenol ( ${C_6}{H_5}OH$ ) is considered as weak acid because its aqueous solution is weakly acidic in nature with pH value $5$ to $6$ but is acidity is not enough to turn blue litmus paper red whereas Sodium hydroxide (NaOH) is a strong base.
So, we can say that the reaction between phenol and sodium hydroxide is an acid-base reaction which is also known as neutralisation reaction.
We know that, when an acid reacts with a base, the products formed are a salt and a water molecule.
Reaction of phenol with sodium hydroxide (NaOH) is given as follows:
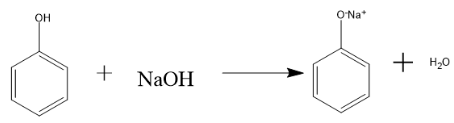
Hence, in the above reaction first the strong base NaOH abstract the proton of phenol molecule and then the ion(mild acid) reacts with sodium hydroxide (base) to form sodium phenoxide which is a colourless solution and water molecule,
Therefore, when Phenol reacts with NaOH sodium phenoxide, (a salt) and a water molecule is formed.
Note:
Phenol is also known as carbolic acid and it is an aromatic compound with chemical formula ${C_6}{H_5}OH$ . It is mildly acidic in nature and can react with a base showing its acidic property while Sodium hydroxide is also known as caustic soda and have chemical formula NaOH and is strongly alkaline in nature.
Complete answer:
Phenol ( ${C_6}{H_5}OH$ ) is considered as weak acid because its aqueous solution is weakly acidic in nature with pH value $5$ to $6$ but is acidity is not enough to turn blue litmus paper red whereas Sodium hydroxide (NaOH) is a strong base.
So, we can say that the reaction between phenol and sodium hydroxide is an acid-base reaction which is also known as neutralisation reaction.
We know that, when an acid reacts with a base, the products formed are a salt and a water molecule.
Reaction of phenol with sodium hydroxide (NaOH) is given as follows:
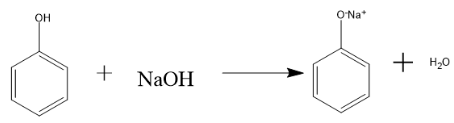
Hence, in the above reaction first the strong base NaOH abstract the proton of phenol molecule and then the ion(mild acid) reacts with sodium hydroxide (base) to form sodium phenoxide which is a colourless solution and water molecule,
Therefore, when Phenol reacts with NaOH sodium phenoxide, (a salt) and a water molecule is formed.
Note:
Phenol is also known as carbolic acid and it is an aromatic compound with chemical formula ${C_6}{H_5}OH$ . It is mildly acidic in nature and can react with a base showing its acidic property while Sodium hydroxide is also known as caustic soda and have chemical formula NaOH and is strongly alkaline in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




