
When HBr adds on hex-1-ene in the presence of benzoyl peroxide, the product is
(A) 1-bromohexane
(B) 2,3-dibromohexane
(C) 1,2- dibromohexane
(D) 2,4-dibromohexane
Answer
587.7k+ views
Hint: Whenever there is an addition reaction in presence of peroxide, the reaction proceeds through formation of radical intermediate. Stability of intermediate decides the final product of the reaction. In the presence of sunlight heterolytic cleavage takes place.
Complete step by step answer:
Reaction of alkene with HBr results in addition reaction. Addition reaction in presence of peroxide proceeds through formation of radical intermediate. In the presence of peroxide, anti-markovnikov's addition takes place. In this reaction, Benzoyl peroxide acts as initiator, which initiates the reaction.

Benzoyl peroxide on heterolytic cleavage leads to formation of free radicals which act as an initiator.
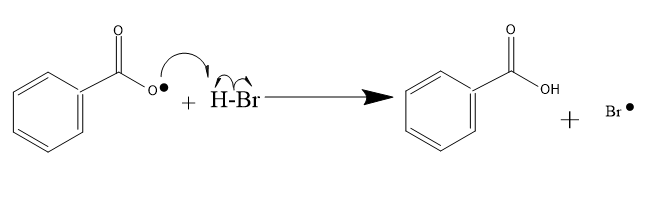
This free radical cleaves the HBr bond and the free radical of the benzoyl group takes up the hydrogen , and the Bromine atom with a free radical is left to react with the hexene.
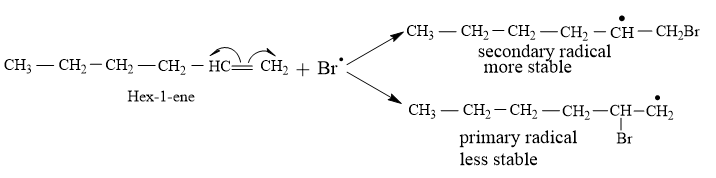
Hexene, in presence of Bromine free radical, cleaves the double bond homolytically, leading to formation of two free radical intermediates. One intermediate has secondary free radical, and bromine atom attaches to the terminal carbon atom, and other intermediate have primary free radical, with bromine atom added to the carbon at second position. As we know, that secondary free radical is more stable than primary free radical, therefore, intermediate having secondary free radical is the stable one and this intermediate will lead to the formation of the product.
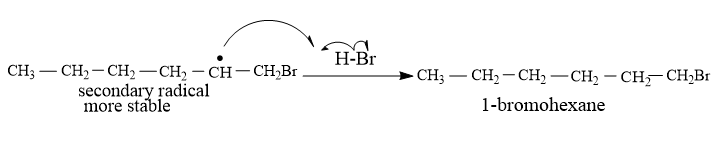
To the secondary free radical, another molecule of HBr reacts, and Hydrogen atoms get attached to the carbon with radical.This leads to the formation of a more stable product through anti-markovnikov's addition.
 So, the correct answer is “Option A”.
So, the correct answer is “Option A”.
Note: In absence of peroxide, the addition is through markovnikov's addition, and proceeds through the formation of carbocation as an intermediate. The end product is decided by the stability of carbocation intermediate forms.
Complete step by step answer:
Reaction of alkene with HBr results in addition reaction. Addition reaction in presence of peroxide proceeds through formation of radical intermediate. In the presence of peroxide, anti-markovnikov's addition takes place. In this reaction, Benzoyl peroxide acts as initiator, which initiates the reaction.

Benzoyl peroxide on heterolytic cleavage leads to formation of free radicals which act as an initiator.

This free radical cleaves the HBr bond and the free radical of the benzoyl group takes up the hydrogen , and the Bromine atom with a free radical is left to react with the hexene.
Hexene, in presence of Bromine free radical, cleaves the double bond homolytically, leading to formation of two free radical intermediates. One intermediate has secondary free radical, and bromine atom attaches to the terminal carbon atom, and other intermediate have primary free radical, with bromine atom added to the carbon at second position. As we know, that secondary free radical is more stable than primary free radical, therefore, intermediate having secondary free radical is the stable one and this intermediate will lead to the formation of the product.

To the secondary free radical, another molecule of HBr reacts, and Hydrogen atoms get attached to the carbon with radical.This leads to the formation of a more stable product through anti-markovnikov's addition.

Note: In absence of peroxide, the addition is through markovnikov's addition, and proceeds through the formation of carbocation as an intermediate. The end product is decided by the stability of carbocation intermediate forms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




