
Here, $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ displays:
A.One $Co - Co$ bond, six terminal $CO$ and two bridging $CO$
B.One $Co - Co$ bond, four terminal $CO$ and four bridging $CO$
C.No $Co - Co$ bond, six terminal $CO$ and two bridging $CO$
D.No $Co - Co$ bond, four terminal $CO$ and four bridging $CO$
Answer
519.3k+ views
Hint: We have to know that, the di-cobalt octa-carbonyl is a white strong when of high immaculateness, however more regularly is an orange-hued, pyrophoric strong that is thermally precarious. It is incorporated by the high-pressure carbonylation of cobalt $\left( {II} \right)$ salts.
Complete answer:
We have to know that the given $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ compound name is Di-cobalt octa carbonyl. This di-cobalt octa carbonyl is the organometallic compound. This metal carbonyl is utilized as a reagent and impetus in organometallic science and natural combination and is integral to much-known organo-cobalt science. It is the antecedent to a hydroformylation impetus, cobalt tetracarbonyl hydride. Every particle comprises two cobalt molecules bound to eight carbon monoxide ligands, however numerous unmistakable primary courses of action have to be known. When, a portion of the carbonyl-ligand is exceptionally labile. The compound is profoundly responsive towards alkynes and is now and again utilized as an alkyne securing bunch. The coordination number of $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ is five.
The structure of the $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ has to be drawn below,
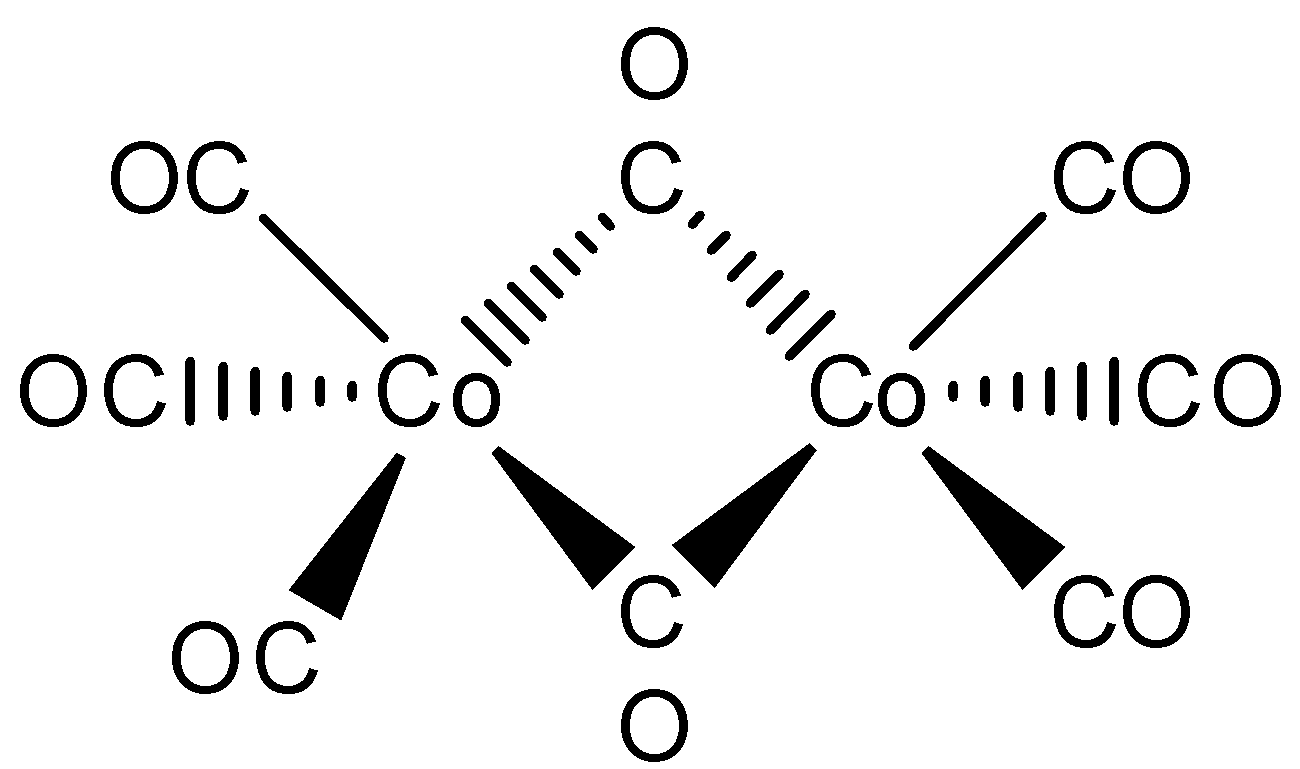
Here, the given metal is cobalt. In this above structure, one $Co - Co$ metal bond is connected with eight carbonyl ligands. Where, two carbonyl ligands are bonded with bridging and remaining six carbonyl ligands are bonded with terminal. So that the correct sentence is, One $Co - Co$ bond, six terminal $CO$ and two bridging $CO$ .
Hence, option (A) is correct.
Note:
We have to know that, $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ an unstable wellspring of cobalt $\left( 0 \right)$ , is pyrophoric and deliveries carbon monoxide upon decay. The National Institute for Occupational Safety and Health has suggested that specialists ought not be presented to focuses more prominent than $0.1mg/{m^3}$ throughout an eight-hour time-weighted normal, without the legitimate respiratory stuff.
Complete answer:
We have to know that the given $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ compound name is Di-cobalt octa carbonyl. This di-cobalt octa carbonyl is the organometallic compound. This metal carbonyl is utilized as a reagent and impetus in organometallic science and natural combination and is integral to much-known organo-cobalt science. It is the antecedent to a hydroformylation impetus, cobalt tetracarbonyl hydride. Every particle comprises two cobalt molecules bound to eight carbon monoxide ligands, however numerous unmistakable primary courses of action have to be known. When, a portion of the carbonyl-ligand is exceptionally labile. The compound is profoundly responsive towards alkynes and is now and again utilized as an alkyne securing bunch. The coordination number of $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ is five.
The structure of the $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ has to be drawn below,

Here, the given metal is cobalt. In this above structure, one $Co - Co$ metal bond is connected with eight carbonyl ligands. Where, two carbonyl ligands are bonded with bridging and remaining six carbonyl ligands are bonded with terminal. So that the correct sentence is, One $Co - Co$ bond, six terminal $CO$ and two bridging $CO$ .
Hence, option (A) is correct.
Note:
We have to know that, $\left[ {C{o_2}{{\left( {CO} \right)}_8}} \right]$ an unstable wellspring of cobalt $\left( 0 \right)$ , is pyrophoric and deliveries carbon monoxide upon decay. The National Institute for Occupational Safety and Health has suggested that specialists ought not be presented to focuses more prominent than $0.1mg/{m^3}$ throughout an eight-hour time-weighted normal, without the legitimate respiratory stuff.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




