
Here,\[3 - methyl - 2 - butnol\] on treatment with $HCl$ gives predominantly:
a) \[2 - chloro - 2 - methylbutane\]
b) \[2 - chloro - 3 - methylbutane\]
c) \[2,2 - dimethylpentane\]
d) None of these
Answer
519.3k+ views
Hint: In order to answer this question we have to draw the structure of the given compound \[3 - methyl - 2 - butanol\] and on treatment with hydrochloric acid a carbocation is formed as a product after that note the stability of the formed carbocation and rearrange it.
Complete answer:
We can write the reaction of \[3 - methyl - 2 - butanol\] with hydrochloric acid is as follows,
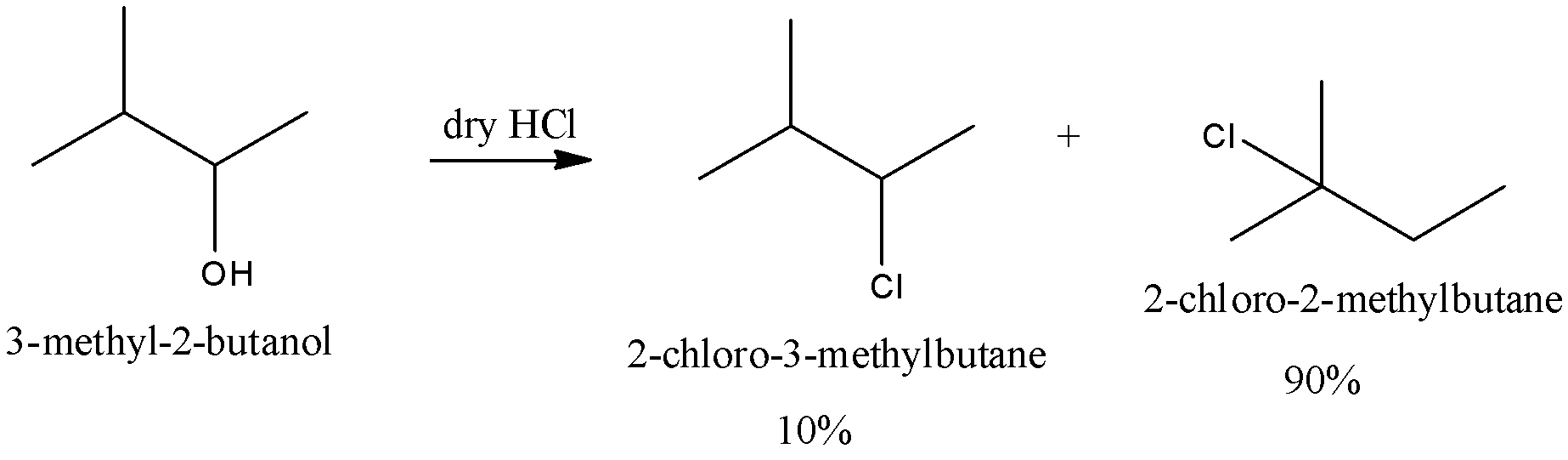
The mechanism of the reaction is discussed below,
Step 1: Protonation is otherwise named as hydro nation which means addition of hydrogen or proton to an atom and these results in the formation of conjugate acid of the mentioned compound. Here, the given \[3 - methyl - 2 - butanol\] gains a pair of hydrogen cations and gets attached to the hydroxyl ion.
Step 2: In this step a carbocation is formed. We know that carbocation is a carbon molecule with three positive charges. Carbonium ion is the other name of carbocation. After the proton is attached to hydroxyl ion a secondary carbocation is formed due to the elimination of water molecules.
Step 3: After a water molecule is eliminated from a compound there is a hydride ion shift. The shift of hydride ions is a type of the rearrangement of carbocation. In this type the hydrogen atom present in the carbocation replaces the carbon atom with formal charge $ + 1$ from a neighbour carbon atom.
Step 4: After the shift of hydride ion a tertiary carbocation is formed as a result and it is the most stable carbocation of all. Then, the chlorine molecule attacks the formed stable carbocation and as a result \[2 - chloro - 2 - methylbutane\] is formed as a major product.
Hence option A is correct.
Note:
We have to remember that the \[S{N^1}\] response is a nucleophilic replacement response in which the rate deciding advance is unimolecular. It is a sort of natural replacement response. \[S{N^1}\] represents replacement nucleophilic unimolecular. Along these lines, the rate condition holds in circumstances where the measure of the nucleophile is far more prominent than the measure of the carbocation in the middle of the path of the reaction.
Complete answer:
We can write the reaction of \[3 - methyl - 2 - butanol\] with hydrochloric acid is as follows,

The mechanism of the reaction is discussed below,
Step 1: Protonation is otherwise named as hydro nation which means addition of hydrogen or proton to an atom and these results in the formation of conjugate acid of the mentioned compound. Here, the given \[3 - methyl - 2 - butanol\] gains a pair of hydrogen cations and gets attached to the hydroxyl ion.
Step 2: In this step a carbocation is formed. We know that carbocation is a carbon molecule with three positive charges. Carbonium ion is the other name of carbocation. After the proton is attached to hydroxyl ion a secondary carbocation is formed due to the elimination of water molecules.
Step 3: After a water molecule is eliminated from a compound there is a hydride ion shift. The shift of hydride ions is a type of the rearrangement of carbocation. In this type the hydrogen atom present in the carbocation replaces the carbon atom with formal charge $ + 1$ from a neighbour carbon atom.
Step 4: After the shift of hydride ion a tertiary carbocation is formed as a result and it is the most stable carbocation of all. Then, the chlorine molecule attacks the formed stable carbocation and as a result \[2 - chloro - 2 - methylbutane\] is formed as a major product.
Hence option A is correct.
Note:
We have to remember that the \[S{N^1}\] response is a nucleophilic replacement response in which the rate deciding advance is unimolecular. It is a sort of natural replacement response. \[S{N^1}\] represents replacement nucleophilic unimolecular. Along these lines, the rate condition holds in circumstances where the measure of the nucleophile is far more prominent than the measure of the carbocation in the middle of the path of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




