
How can you test for ketones?
Answer
555.3k+ views
Hint:The ketones are the carbonyl compound generally identified by $R - O - R$. The ketone can be represented by the suffix- one. For example: acetone. The ketone can be tested mainly by two chemical tests 2, 4-dinitrophenylhydrazine test and Sodium bisulfite test.
Complete step by step answer:The ketones are the functional group which are represented by $R - O - R$, where R is the alkyl group. It belongs to a carbonyl group with aldehyde. The oxygen atom is bonded with the alkyl carbon by a double bond. The ketone is represented by the suffix-one.
The carbonyl group consisting of the ketones and aldehyde are tested by various tests like 2, 4-dinitrophenylhydrazine test and Sodium bisulfite test.
2, 4-dinitrophenylhydrazine test:
Ketones reacts with 2, 4-dinitrophenyl hydrazine to give yellow to orange precipitate.
The reaction between the ketone compound and 2, 4-dinitrophenyl hydrazine is shown below.
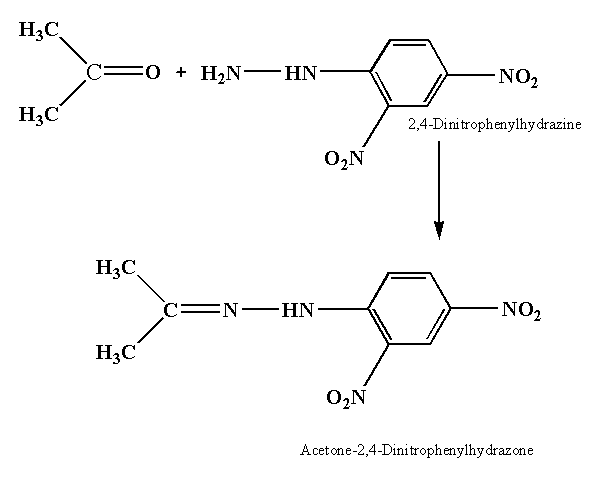
In this reaction acetone reacts with 2,4-Dinitrophenylhydrazine to give yellow precipitate of acetone-2,4-Dinitrophenylhydrazone.
Formation of yellow precipitate indicates the presence of ketone.
Sodium bisulfite test:
The ketone compound reacts with sodium bisulfite to form a product known as ketone bisulfite which is soluble in water.
The chemical reaction between the ketone compound and sodium bisulfite is shown below.
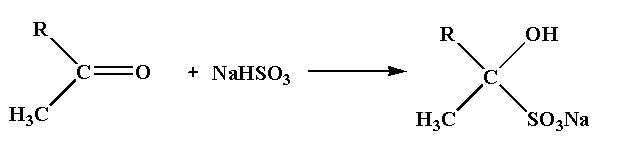
In this reaction, the ketone compound reacts with the sodium bisulfite to form a complex ketone bisulfite.
The presence of white crystalline precipitate confirms the presence of ketone.
Note:
The ketone can be distinguished from aldehyde by using different chemical tests. One of the tests is known as Tollen’s test also known as silver mirror test. The Tollen’s reagent contains silver ammonia complex in ammonia solution which on reacting with aldehyde gives grey-black precipitate or silver mirror. The aldehydic compound gets oxidized to acid and silver present in the complex changes its state from +1 to its elemental state. The ketone compound does not give a silver mirror test.
The reaction is shown below.
$R - CHO + 2Cr{O_3} + 3{H_2}S{O_4} \to 3R - C(O) - OH + 3{H_2}O + C{r_2}{(S{O_4})_3}$
The presence of green precipitate confirms the presence of aldehyde.
Complete step by step answer:The ketones are the functional group which are represented by $R - O - R$, where R is the alkyl group. It belongs to a carbonyl group with aldehyde. The oxygen atom is bonded with the alkyl carbon by a double bond. The ketone is represented by the suffix-one.
The carbonyl group consisting of the ketones and aldehyde are tested by various tests like 2, 4-dinitrophenylhydrazine test and Sodium bisulfite test.
2, 4-dinitrophenylhydrazine test:
Ketones reacts with 2, 4-dinitrophenyl hydrazine to give yellow to orange precipitate.
The reaction between the ketone compound and 2, 4-dinitrophenyl hydrazine is shown below.

In this reaction acetone reacts with 2,4-Dinitrophenylhydrazine to give yellow precipitate of acetone-2,4-Dinitrophenylhydrazone.
Formation of yellow precipitate indicates the presence of ketone.
Sodium bisulfite test:
The ketone compound reacts with sodium bisulfite to form a product known as ketone bisulfite which is soluble in water.
The chemical reaction between the ketone compound and sodium bisulfite is shown below.

In this reaction, the ketone compound reacts with the sodium bisulfite to form a complex ketone bisulfite.
The presence of white crystalline precipitate confirms the presence of ketone.
Note:
The ketone can be distinguished from aldehyde by using different chemical tests. One of the tests is known as Tollen’s test also known as silver mirror test. The Tollen’s reagent contains silver ammonia complex in ammonia solution which on reacting with aldehyde gives grey-black precipitate or silver mirror. The aldehydic compound gets oxidized to acid and silver present in the complex changes its state from +1 to its elemental state. The ketone compound does not give a silver mirror test.
The reaction is shown below.
$R - CHO + 2Cr{O_3} + 3{H_2}S{O_4} \to 3R - C(O) - OH + 3{H_2}O + C{r_2}{(S{O_4})_3}$
The presence of green precipitate confirms the presence of aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




