
How do you name benzene derivatives?
Answer
534k+ views
Hint: Benzene derivatives are the compounds formed by electrophilic substitution of the benzene ring and they are named by using substituents as a prefix to the benzene and in the case of more than one group, the least possible numbers should be assigned to the groups. The nomenclature of benzene derivatives is a bit complicated due to the different common names of each compound.
Complete answer:
Benzene is the representative member of a class of aromatic organic compounds and it has a molecular formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}$. It is a six-membered ring where each carbon is attached to one hydrogen atom and there are three alternate double bonds in the ring which satisfy the tetravalency of carbons.
The benzene ring tends to undergo electrophilic substitution reactions. This results in the formation of a large number of benzene derivatives in which one or more hydrogens get replaced by other functional groups.
Let us discuss the nomenclature of aromatic compounds.
(1) – Monosubstituted benzenes.
The benzene compounds containing a single substituent on their ring are named by adding the substituent’s name as a prefix to the benzene. For example, when a chlorine group is attached to the benzene ring, we name it Chlorobenzene.
Similarly, when a nitro group is attached, the nomenclature would be Nitrobenzene and for a methyl group attached to the ring, the name would be Methylbenzene. But methylbenzene is commonly called toluene.
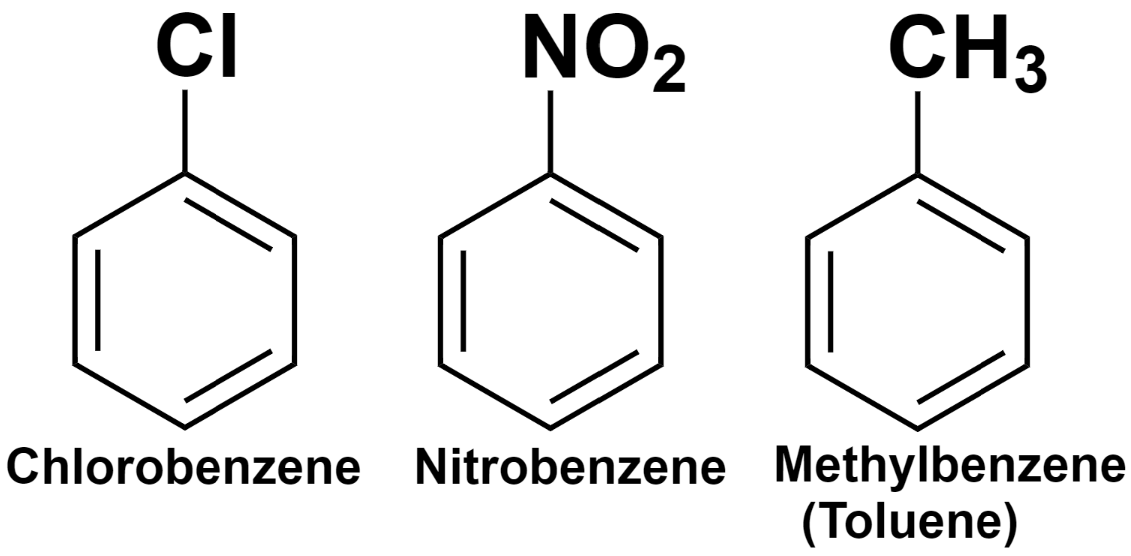
(2) – Disubstituted benzenes
There are three possible structures for disubstituted benzenes. They are designated by using prefixes: ortho- (1,2-), para- (1,3-), meta- (1,4-) as described below.
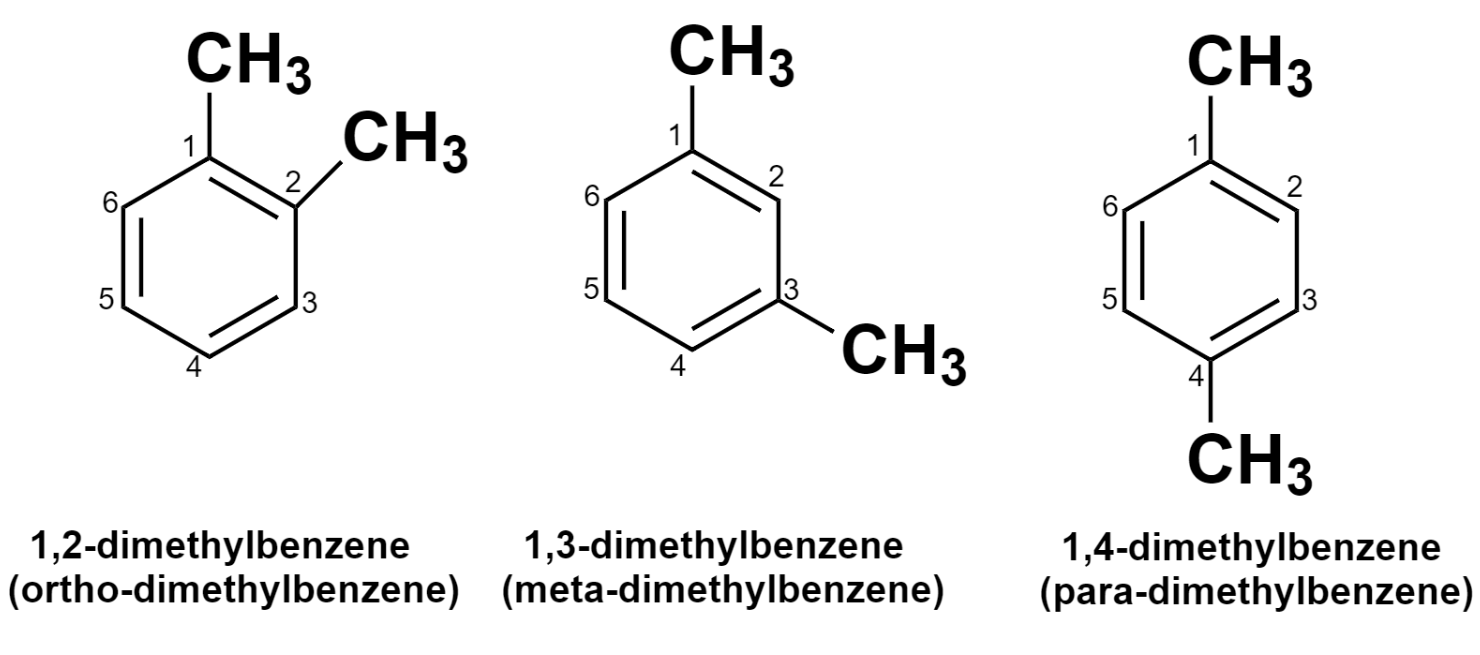
(3) – Polysubstituted benzenes
When more than two substituents are attached to different points in the ring, then their positions are designated by numbering the carbon atoms of the benzene ring in such a manner that each substituent will get the minimum possible number. Look at the below diagram for a better understanding.
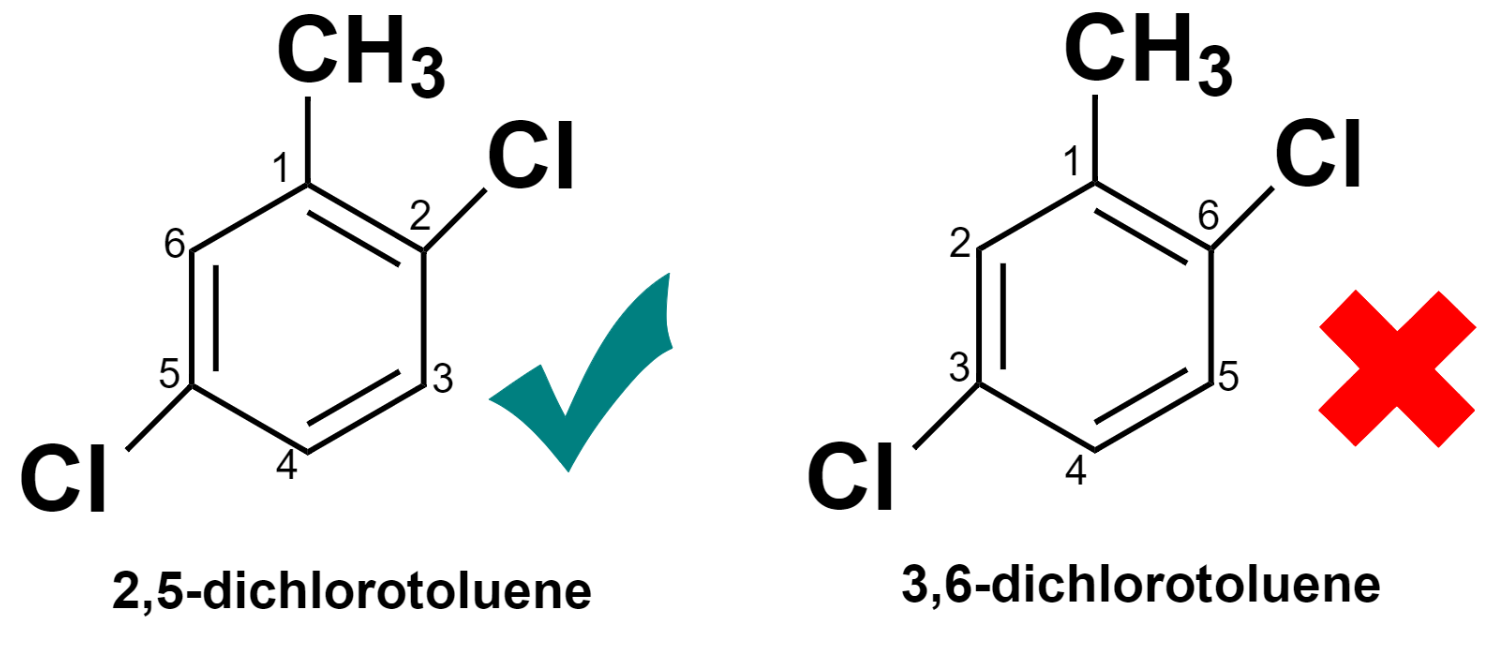
(4) – When two different groups are present, they should be named in alphabetical order.
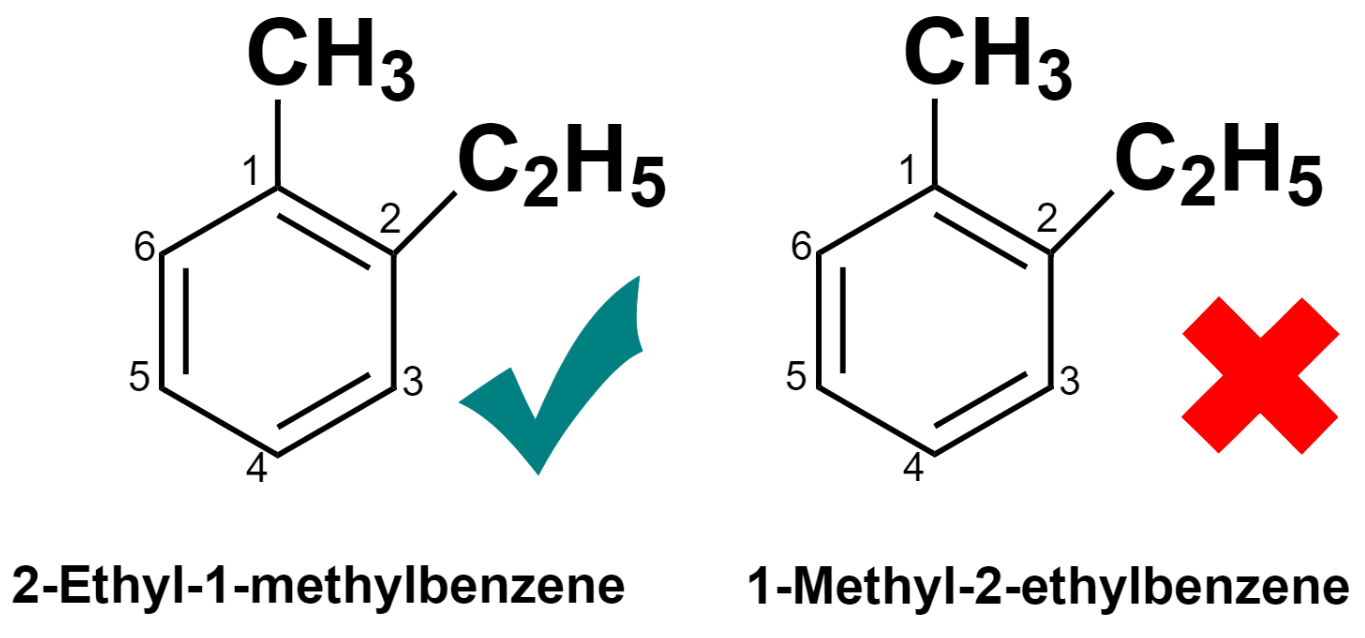
(5) – When the benzene ring is present as a substitute group, it is named phenyl.
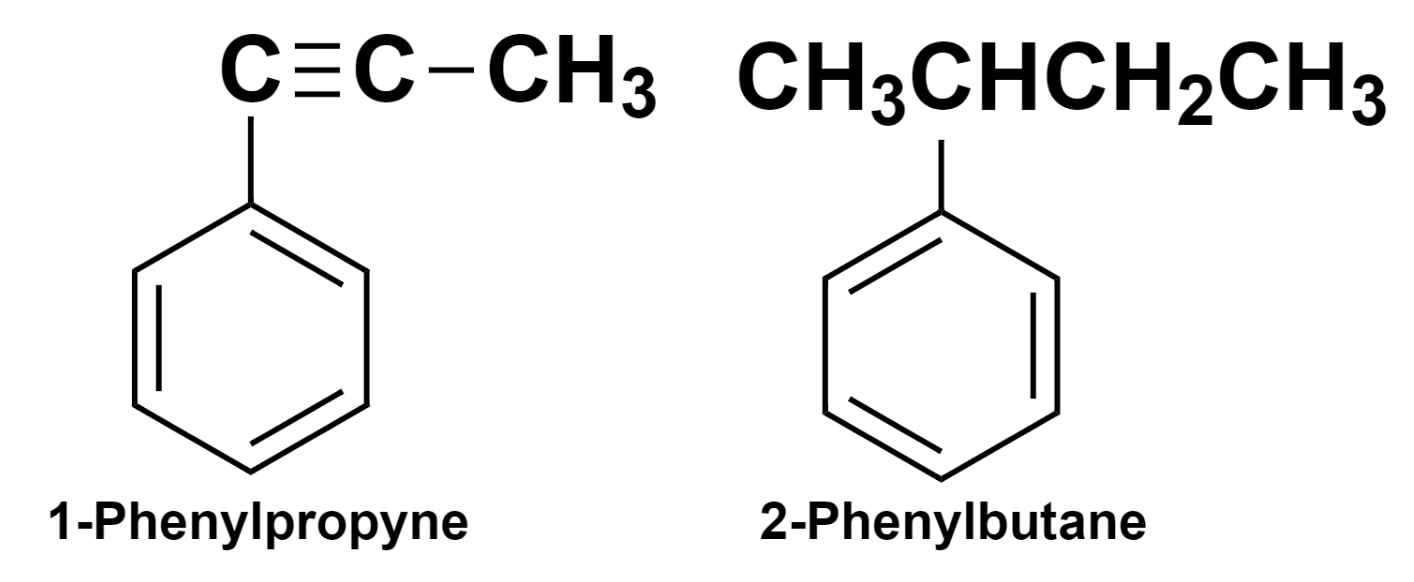
Hence, the benzene derivatives can be named in this way.
Note:
During the nomenclature of benzene derivatives, the numbering of the ring can be done either in a clockwise or anticlockwise direction keeping in mind that the lowest possible number is assigned to the substituent groups.
Complete answer:
Benzene is the representative member of a class of aromatic organic compounds and it has a molecular formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}$. It is a six-membered ring where each carbon is attached to one hydrogen atom and there are three alternate double bonds in the ring which satisfy the tetravalency of carbons.
The benzene ring tends to undergo electrophilic substitution reactions. This results in the formation of a large number of benzene derivatives in which one or more hydrogens get replaced by other functional groups.
Let us discuss the nomenclature of aromatic compounds.
(1) – Monosubstituted benzenes.
The benzene compounds containing a single substituent on their ring are named by adding the substituent’s name as a prefix to the benzene. For example, when a chlorine group is attached to the benzene ring, we name it Chlorobenzene.
Similarly, when a nitro group is attached, the nomenclature would be Nitrobenzene and for a methyl group attached to the ring, the name would be Methylbenzene. But methylbenzene is commonly called toluene.

(2) – Disubstituted benzenes
There are three possible structures for disubstituted benzenes. They are designated by using prefixes: ortho- (1,2-), para- (1,3-), meta- (1,4-) as described below.

(3) – Polysubstituted benzenes
When more than two substituents are attached to different points in the ring, then their positions are designated by numbering the carbon atoms of the benzene ring in such a manner that each substituent will get the minimum possible number. Look at the below diagram for a better understanding.

(4) – When two different groups are present, they should be named in alphabetical order.

(5) – When the benzene ring is present as a substitute group, it is named phenyl.

Hence, the benzene derivatives can be named in this way.
Note:
During the nomenclature of benzene derivatives, the numbering of the ring can be done either in a clockwise or anticlockwise direction keeping in mind that the lowest possible number is assigned to the substituent groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




